Abstract
Background and Aims
Recently, cholera vaccine use was shown to be associated with a reduced risk of death in patients with colorectal cancer (CRC). However, evidence on heterologous effects of travel vaccines is limited. The aim of this study was to study heterologous effects of travel vaccines in patients with CRC.
Methods
We performed a retrospective database study on a cohort of CRC patients in Sweden and their postdiagnostic use of travel medications between July 2005 and December 2017. We obtained data from national registries on number of CRC diagnosis, death from CRC or other causes, age at diagnosis, and postdiagnostic use of travel vaccines and malaria prophylaxis. The Cox regression model was used to calculate incidence rate and incidence rate ratios of CRC-related and all-cause mortality by postdiagnostic travel medication status.
Results
Two hundred ninety-five patients exposed to travel vaccines and malaria prophylaxis and 73,466 patients not exposed to travel medications were identified. CRC-related mortality was lowered in the exposed patients compared to the unexposed patients, irrespective of the travel medications used. The incidence rate ratios for CRC-related mortality and overall mortality were comparable.
Conclusion
We postulated that patients in better health were likely to travel more frequently than patients with poor health, leading to a healthy user bias. The results suggested the same, as similar reduced mortality risks were found for all the investigated travel medications, lowering the biological plausibility of truly protective effect from post-therapeutic use of any of the travel medication studied. We advocate the use of multiple negative exposure controls and to exercise caution while drawing conclusions from travel vaccine research.
Keywords: Dukoral, Colorectal Cancer, Healthy User Bias, Travel Vaccines
Introduction
As the number of people traveling internationally continues to grow, the importance of protecting the health of individual travelers, as well as safeguarding the health of the communities to which they return, cannot be understated.1,2 Between 43% and 79% travelers reported falling ill when traveling in emerging economies.3 Travelers are as unique as their itineraries; they cover all age ranges and might also have pre-existing health problems and conditions.4 The recommendations for vaccination vary depending on the destination and on the general health of the travelers. Most common travel vaccines recommended by the World Health Organization and Centers for Disease Control and Prevention are the ones against cholera, hepatitis A, hepatitis E, Japanese encephalitis, meningococcus, polio (adult booster dose), rabies, tick-borne encephalitis, typhoid fever, and yellow fever when people travel to these disease-endemic countries.2,4 Furthermore, it is recommended to confirm if measles, mumps, rubella and tetanus, diphtheria, and pertussis vaccines were received, if information on immunization was unavailable.
To facilitate the assessment between benefits and risks of vaccine use, extensive efforts are undertaken to evaluate the safety of vaccines (including travel vaccines) from early development throughout its entire duration of use. At licensure, surveillance activities are put into place to continue monitoring the benefits and safety of the vaccines under large-scale and routine use conditions.5,6 Though this process applies for travel vaccines as well, there are very few epidemiological studies on the effects of travel vaccines under such routine use.7
Studying travel vaccines in routine use is challenging as patients in better health conditions are likely to travel more frequently than patients with poorer health conditions. This leads to a healthy user bias,8 which might seriously jeopardize the validity of the results from epidemiological studies on the safety and effectiveness of travel vaccines and medication.9
In a retrospective cohort study of colorectal cancer (CRC) patients in Sweden, Ji et al10 found that the patients with CRC who received the oral cholera vaccine (Dukoral, Valneva Sweden AB) after cancer diagnosis had a decreased risk of death from CRC and decreased risk of death overall. Ji et al found similar effects of the oral cholera vaccine on prostate cancer and breast cancer.11,12 Such nonintended effects of vaccination that go beyond the targeted diseases are known as nonspecific or heterologous effects, and they can be beneficial, neutral, or deleterious.13 Studies from a small number of low-income countries in West Africa reported that the live attenuated tuberculosis vaccine, Bacillus Calmette Guerin, and the measles, mumps, rubella vaccine reduced all-cause mortality, much more than that is expected from the target disease alone14,15; the inactivated tetanus, diphtheria, and pertussis vaccine increased the all-cause mortality.16 Studies on heterologous infections in high-income settings have also shown mixed results.17,18 All these studies assessed the hypothesis that receiving an inactivated vaccine after a live vaccine can be detrimental/harmful as compared to receiving only live vaccine. This hypothesis, however, remains controversial and has not been confirmed unequivocally by further studies.16 Before studies from Ji et al, there was a lack/paucity of such studies on heterologous effects of travel vaccines.
The primary objective of this research was to study the potential effects of travel vaccines on CRC and all-cause mortality in a cohort of CRC patients in Sweden as Ji et al,10 to confirm their work for an extended study period and to include a broader range of travel vaccines (cholera vaccine, hepatitis A/B, hepatitis A, hepatitis B, Japanese encephalitis, rabies, typhoid fever, and yellow fever) to see if these travel vaccines also show similar heterologous effects.
Materials and Methods
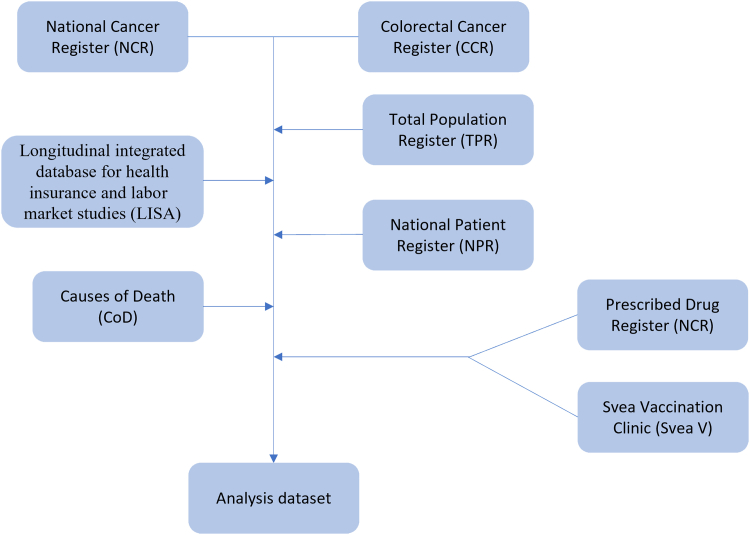
This was a retrospective cohort study on CRC patients in Sweden. Information for this study was obtained from several linked registries: Total Population Register, National Cancer Register (NCR), Colorectal Cancer Register (CCR), Prescribed Drug Register, causes of death register, National Patient Register, longitudinal integrated database for health insurance and labor market studies, and Svea Vaccination Clinic (Table 1). A flow chart that explains how the data sets are linked is presented as Figure 1.
Table 1.
Sources of Data
| Register | Variables |
|---|---|
| Total Population Register (TPR) | Date of birth, country of birth, sex, last migration status, education level, and year education level obtained |
| National Cancer Register (NCR) | Date of diagnosis, ICD-10, morphology, stage of disease |
| Colorectal cancer register (CCR) | Date of diagnosis, ICD-10, information on disease specific covariates |
| Prescribed Drug Register (PDR) | Prescription dispensation date, ATC code |
| Causes of death (CoD) | Date of death, underlying cause of death |
| National Patient Register (NPR) | Hospitalizations, date of admission, date of discharge, ICD-10 |
| Longitudinal integrated database for health insurance and labor market studies (LISA) | Information on disposable income |
| Svea Vaccination Clinic (Svea V) | Prescription dispensation date and ATC codes for the vaccines |
ATC, Anatomical Therapeutic Chemical; ICD, International Classification of Disease.
Figure 1.
A flow chart showing how various data sets are linked.
We searched the NCR and the CCR for patients diagnosed with CRC from July 2005 to December 2017, using the 10th International Classification of Disease19 codes C18, C19, and C20. The outcomes of interest in this study were CRC-related mortality and overall mortality. Information on death due to CRC or any other causes was obtained from the causes of death register. The NCR also records the stages of the cancer based on the TNM system where T describes the size of the tumor, N describes the spread to the nearby lymph nodes, and M describes about the metastasis of the cancer to other parts of the body. Stage 1, using the TNM system, is the least advanced and stage 4 is the most advanced cancer stage.20
The exposures of interest in this study were the administration of antimalarial drugs and travel vaccines (cholera vaccine, hepatitis A/B, hepatitis A, hepatitis B, Japanese encephalitis, rabies, typhoid fever, and yellow fever). For antimalarial drugs, only Malarone and Mefloquine were considered, as the other antimalarial drugs are also used therapeutically (eg, chloroquine is used in the treatment of lupus, an autoimmune disorder).21 Information on the use of travel vaccines and antimalarial drugs in CRC patients was obtained from the Prescribed Drug Register and databases from the Svea Vaccine Clinic (chain of travel vaccination clinics in Sweden), using the Anatomical Therapeutic Chemical codes (Table A1).
The covariates of interest to this study were obtained from the Total Population Register, National Patient Register, longitudinal integrated database for health insurance and labor market studies, and CCR. The covariates included age at diagnosis, sex, country of birth (Sweden, rest of Europe, or others), region (urban vs rural), years of education (<10 years, 11–12 years, or >12 years), family income (first to fourth quartile), tumor stage at diagnosis (stages I–IV), tumor morphology (adenocarcinoma or others), tumor location (colon or rectum), intent of treatment (curative, palliative, unsure, or missing), and hospitalizations ( no or 1–5 days).
Data from the registries mentioned above were linked at the individual level by means of the national personal identification numbers. After linkage completion, the personal identification number was replaced by a study identity number to protect the integrity and maintain the anonymity of personal data.
The start of individual follow-up was from the first registered date of CRC diagnosis from July 1, 2005, and follow-up ended on the date of emigration, death, disease recurrence, or end of the study period (December 31, 2017), whichever occurred first. Individuals were excluded from the study when the date of disease diagnosis was prior to the start of follow-up, when they emigrated before the date of diagnosis, or when they died.
Statistical Analysis
A descriptive characterization of the patient population was performed to identify any systematic differences by vaccination status (defined as being vaccinated with at least one of the travel vaccines used in the study during the follow-up time).
The Cox regression model was used to calculate incidence rates (IRs) and 95% confidence intervals (CIs) for CRC mortality and all-cause mortality as well as incidence rate ratios (IRRs) and their 95% CIs associated with postdiagnostic use of any of the travel medications (Table A1). Potential confounding was controlled by including the following covariates in the regression model: age at CRC diagnosis (continuous variable), year at CRC diagnosis (continuous variable), gender, stage of CRC (stage I–IV), tumor morphology, recurrence, country of birth (Sweden, other European countries, and others), highest educational level (<10, 10–11, or >12 years of education), and family income (categorized per quartile). The date of vaccination/antimalarial drug use was included as a time-varying exposure. Analogous models were fit using Poisson and quasi-Poisson regression to check for the robustness of sensitivity analyses.
All data management and statistical analyses were performed using R 3.6.1.22
All authors had access to the study data and reviewed and approved the final manuscript.
Results
Overview of Baseline Characteristics
A total of 295 CRC patients who were exposed to any of the travel vaccines and malaria prophylaxis of interest between July 2005 and December 2017 were identified. We also identified 73,466 CRC patients without travel medication exposure in the same period. The median age at diagnosis was 73 years for the unexposed and 62 years for the exposed. The age group with the highest percentage of exposed CRC patients was 60–69 years (45.1%), whereas the highest percentage in unexposed individuals was in 70–79 years (33.4%). Of the CRC patients, half were males (52.5%) (Table 2).
Table 2.
Overview of Baseline Characteristics of the Patient Population–CRC Cohort by Exposure to Travel Vaccines/Anti-Malarial Drugs
| Covariates | Unexposed |
Exposed |
||
|---|---|---|---|---|
| N | % | N | % | |
| Cohort size | 73,466 | 99.6 | 295 | 0.4 |
| Follow-up years (mean) | 7.9 | - | 3.7 | - |
| All-cause mortality | 34,028 | 46.3 | 36 | 12.2 |
| CRC-related mortality | 25,224 | 34.3 | 27 | 9.2 |
| Age at CRC diagnosis | ||||
| <50 y | 3900 | 5.3 | 49 | 16.6 |
| 50–59 y | 7236 | 9.8 | 69 | 23.4 |
| 60–69 y | 18,068 | 24.6 | 133 | 45.1 |
| ≥70 y | 44,262 | 60.2 | 44 | 15 |
| Sex | ||||
| Men | 38,581 | 52.5 | 155 | 52.5 |
| Women | 34,885 | 47.5 | 140 | 47.5 |
| Family income | ||||
| First quartile | 18,473 | 25.1 | 26 | 8.8 |
| Second quartile | 18,440 | 25.1 | 33 | 11.2 |
| Third quartile | 18,339 | 25 | 79 | 26.8 |
| Fourth quartile | 17,928 | 24.4 | 157 | 53.2 |
| Missing | 286 | 0.4 | 0 | 0 |
| Stage of disease at diagnosis (tnm) | ||||
| Stage I | 10,362 | 14.1 | 54 | 18.3 |
| Stage II | 18,203 | 24.8 | 76 | 25.8 |
| Stage III | 19,572 | 26.6 | 94 | 31.9 |
| Stage IV | 14,419 | 19.6 | 16 | 5.4 |
| Not assessed | 10,910 | 14.9 | 55 | 18.6 |
TNM staging in exposed individuals showed that 18.3% had stage I CRC, 25.8% had stage II, 31.9% had stage III, and 5.4% had stage IV (18.6% were not assessed, or values were inconclusive) at the time of diagnosis. In the unexposed individuals, 14.1% had stage I CRC, 24.8% had stage II, 26.6% had stage III, and 19.6% had stage IV (14.9% were not assessed). Adenocarcinomas were the most common type of tumor in both the groups (96.6% in unexposed and 94.0% in the exposed), and the tumor location was also similar in both the groups (colon accounting for 67.0% and 33.0% was rectum). Intent of treatment was curative in 60.6% of unexposed individuals and 72.5% of exposed individuals. A total of 64.0% of the individuals in the exposed group had no hospitalizations in the last 12 months as compared to 52.0% in the unexposed group (Table 2 and Table A2).
A total of 39.3% of the unexposed individuals had less than 10 years of education as compared to 12.0% of exposed individuals. Furthermore, for family income, the percentage of individuals in the 4 quartiles was equally distributed in the unexposed group, whereas in the exposed group, the highest percentage (54.0%) belonged to the fourth quartile (Table 2 and Table A2).
Travel Vaccination and Medications
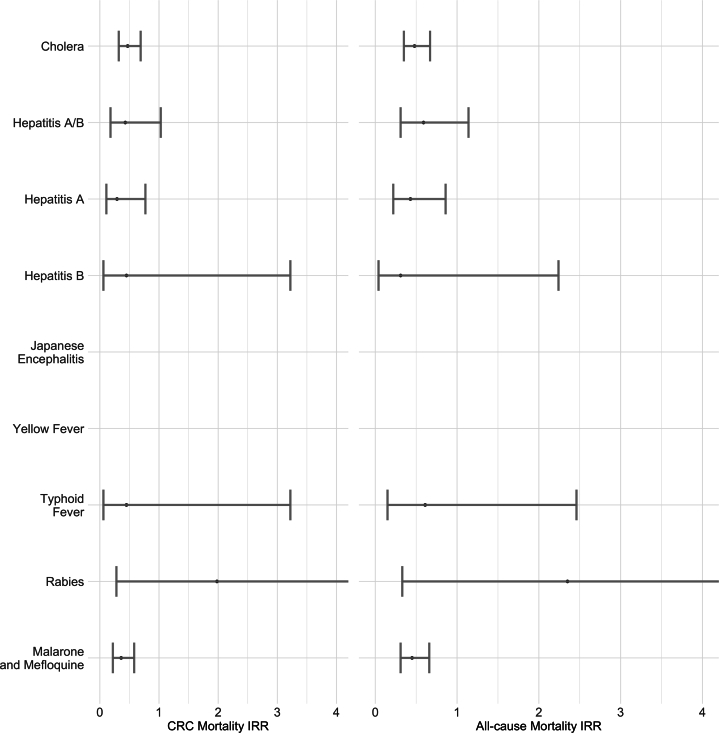
The association between travel vaccines and antimalarial medications and the mortality of CRC patients was expressed in terms of the IR and IRR (95% CI). Figure 2 summarizes the all-cause mortality IRR and CRC-related mortality IRR for all the travel vaccines and medications. The all-cause mortality IRR for the cholera vaccine was 0.48 (0.35–0.67), and the CRC-related mortality was 0.47 (0.32–0.69). For the malarial medications, Malarone and Mefloquine, the all-cause and CRC-related mortality IRRs were 0.45 (0.31–0.66) and 0.36 (0.22–0.58), respectively. There were no pronounced differences between the 2 mortality IRRs for any of the vaccines or medications. The ratios for Japanese encephalitis and yellow fever could not be calculated owing to the small sample sizes. Crude IRs for all-cause mortality and CRC-related mortality for unexposed and exposed groups with respect to years since diagnosis and age at diagnosis are summarized in Figure 2 and Table A3. Crude IRs for overall mortality and CRC-related mortality were lower in the exposed group than those in the unexposed group for both years since diagnosis and the age at diagnosis.
Figure 2.
The association between travel medication and vaccines and the all-cause and CRC-related mortality of CRC patients using the Cox regression model (unmatched cohort and extended covariates) between July 2005 and December 2017. The IRR for Japanese encephalitis and yellow fever was inestimable owing to a small sample size.
Sensitivity analyses are presented in Table A4.
Discussion
This was a retrospective, observational, dynamic cohort study to identify the heterologous effects of postdiagnostic use of travel vaccines and malarial medications in CRC patients in Sweden. Various national registries were used to get the data on CRC, use of travel medications, and demographics of the population. A total of 73,466 individuals were diagnosed with CRC between July 2005 and December 2017; of these, 295 received various travel medications (Table 2) after diagnosis.
Ji et al10 reported that the use of the cholera vaccine reduced the risk of death in patients with CRC in Sweden. We wanted to confirm the heterologous effects of the cholera vaccine as shown by these authors. In our study, we used the same population registries, extended the study period by 2 more years, and used the same vaccine and medications. Additionally, we also included other travel vaccines as well (hepatitis A/B, typhoid fever, yellow fever, Japanese encephalitis, and rabies). Our results (summarized in Table 3) show that there was a trend toward decreased CRC-related mortality with the use of malarial medications and with all the travel vaccines studied. Malarial medications (primarily hydroxychloroquine) are known to have anticancer effects.23 However, our results with travel vaccines, while confirming the results of Ji et al, also showed similar positive heterologous effects for all the travel vaccines and medications studied.
Table 3.
Crude IR of All-Cause Mortality and CRC-Related Mortality by Years Since Diagnosis and Age at Diagnosis
| Covariates | All-cause mortality |
CRC-related mortality |
||||||
|---|---|---|---|---|---|---|---|---|
| Unexposed |
Exposed |
Unexposed |
Exposed |
|||||
| Deaths | Crude IR (95% CI) | Deaths | Crude IR (95% CI) | Deaths | Crude IR (95% CI) | Deaths | Crude IR (95% CI) | |
| Year(s) since diagnosis | ||||||||
| <1 | 13,707 | 219.3 (215.7–223.0) | 1 | 43.1 (1.1–240.1) | 11,743 | 187.9 (184.5–191.3) | 0 | 0 (0–159.0) |
| 1–2 | 6663 | 138.6 (135.3–141.9) | 3 | 32.4 (6.7–94.7) | 5523 | 114.9 (111.9–117.9) | 3 | 32.4 (6.7–94.7) |
| 2–3 | 4033 | 105.6 (102.4–108.9) | 4 | 27.9 (7.6–71.4) | 3052 | 79.9 (77.1–82.8) | 4 | 27.9 (7.6–71.4) |
| 3–4 | 2819 | 91.03 (87.7–94.5) | 5 | 28.5 (9.3–66.5) | 1837 | 59.3 (56.6–62.1) | 5 | 28.5 (9.3–66.5) |
| 4 | 1982 | 79.0 (75.5–82.5) | 5 | 27.0 (8.8–63.1) | 1148 | 45.7 (43.1–48.5) | 3 | 16.2 (3.4–47.4) |
| 5–9 | 4413 | 69.6 (67.6–71.7) | 16 | 21.3 (12.2–34.6) | 1827 | 28.8 (27.5–30.2) | 12 | 16.0 (8.3–27.9) |
| ≥10 | 411 | 71.7 (64.9–79.0) | 2 | 18.54 (2.2–67.0) | 94 | 16.4 (13.3–20.1) | 0 | 0 (0–34.2) |
| Age at diagnosis (y) | ||||||||
| <50 | 1117 | 64.2 (60.5–68.1) | 3 | 13.2 (2.7–38.5) | 1035 | 59.5 (55.9–63.2) | 3 | 13.2 (2.7–38.5) |
| 50–59 | 2313 | 69.5 (66.7–72.3) | 4 | 11.8 (3.2–30.2) | 2051 | 61.6 (59.0–64.3) | 4 | 11.8 (3.2–30.2) |
| 60–69 | 6440 | 82.7 (80.7–84.7) | 21 | 31.2 (19.3–47.7) | 5360 | 68.8 (67.0–70.7) | 15 | 22.3 (12.5–36.8) |
| 70–79 | 11,213 | 122.9 (120.7–125.2) | 6 | 27.3 (10.0–59.5) | 8224 | 90.2 (88.2–92.2) | 5 | 22.8 (7.4–53.2) |
| ≥80 | 12,945 | 239.2 (235.1–243.4) | 2 | 100 | 8554 | 158.1 (154.8–161.5) | 0 | 0 (0–186.2) |
Thus, we postulated that the basic characteristics of people who travel and get vaccinated tend to be different than the people who do not, especially when the travelers have chronic, life-threatening diseases. This leads to a healthy user/healthy vaccinee bias in observational studies of this nature.8 Ji et al11 had accounted for health user bias by propensity matching and confounding bias by the use of antimalarial drugs as negative exposure controls. They reported increased CRC and overall mortality with the antimalarial drugs. However, we have shown that the mortality of exposed CRC patients was reduced with all the travel vaccines and malarial medications studied. These findings lower (though not eliminate) the biological plausibility of any truly protective effect of the post-therapeutic use of any of the travel vaccines/medications studied but might be explained by healthy user/healthy vaccinee bias.
Nonspecific heterologous effects are much more complex than the direct vaccine effects as the underlying biological mechanisms are not always understood, and there can be unknown number of diseases that might be affected. Furthermore, we need to exercise caution against confounding bias in retrospective, observational studies on travel vaccines and medications. To this end, the use of negative exposure controls to detect confounding by indication bias is important and helps in accurate interpretation of results.24, 25, 26 Moreover, it is important to select the negative exposure controls well and preferably use multiple ones, as our study shows.27,28
In this study, our data directly challenge the interpretations of results from Ji et al,10 suggesting a strongly protective effect of postdiagnostic use of Dukoral on mortality in CRC patients. We have shown that we need to exercise caution while interpreting results from an observational study on travel vaccines as healthy user/vaccinee bias might skew the results. Travel vaccines are generally administered in a healthier population, and thus, care must be taken when extrapolating these results to the general population. An appropriate selection of negative exposure controls can help against confounding by indication bias. Furthermore, to thoroughly study the potential effects of heterologous effects of travel vaccines without healthy user/vaccinee bias, conducting prospective, randomized trials and studies with comparable cohorts should be considered.
Acknowledgments
Authors' Contributions:
Eva Herweijer contributed to methodology, data curation, formal analysis, and writing—review and editing. Klaus Schwamborn contributed to conceptualization, methodology, and writing—review and editing. Kaatje Bollaerts contributed to methodology, formal analysis, writing—original draft, and writing—review and editing. Adrian Spillmann contributed to conceptualization, methodology, and writing—review and editing. Tom Cattaert contributed to methodology and formal analysis. Thomas Verstraeten contributed to conceptualization, resources, and project administration. Janet Hoogstraate contributed to conceptualization, supervision, resources, project administration, funding acquisition, and writing—review and editing.
Footnotes
Conflicts of Interest: These authors disclose the following: K.S., A.S., and J.H. are employees of Valneva, the manufacturers of the oral cholera vaccine, Dukoral, used in the study. K.B., E.H., T.C., and T.V.’s employer, P95 Epidemiology & Pharmacovigilance, received funding from Valneva for this work.
Funding: The study was funded by Valneva Sweden AB. Writing assistance was provided by the medical writer at P95 and funded by Valneva.
Ethical Statement: The corresponding author, on behalf of all authors, jointly and severally, certifies that their institution has approved the protocol for any investigation involving humans or animals and that all experimentation was conducted in conformity with ethical and humane principles of research.
Data Transparency Statement: Data cannot be shared. Analytic methods and study materials can be made available upon requests to authors.
Writing Assistance: Archana Nagarajan, Ph.D., (P95 Epidemiology & Pharmacovigilance) provided the medical writing support.
Material associated with this article can be found in the online version at https://doi.org/10.1016/j.gastha.2022.02.013.
Supplementary Materials
References
- 1.World Bank WTO . World Bank Group; 2021. International tourism, number of arrivals.https://data.worldbank.org/indicator/ST.INT.ARVL [Google Scholar]
- 2.Freedman D.O., Chen L.H. Vaccines for international travel. Mayo Clin Proc. 2019;94:2314–2339. doi: 10.1016/j.mayocp.2019.02.025. [DOI] [PubMed] [Google Scholar]
- 3.Angelo K.M., Kozarsky P.E., Ryan E.T., et al. What proportion of international travellers acquire a travel-related illness? A review of the literature. J Travel Med. 2017;24 doi: 10.1093/jtm/tax046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention . Volume 2021. Yellow Book. Centers for Disease Control (CDC); 2020. https://wwwnc.cdc.gov/travel/yellowbook/2020/preparing-international-travelers/the-pretravel-consultation (Chapter 2. Preparing international travelers, vaccination & immunoprophylaxis: general recommendations). [Google Scholar]
- 5.Di Pasquale A., Bonanni P., Garçon N., et al. Vaccine safety evaluation: practical aspects in assessing benefits and risks. Vaccine. 2016;34:6672–6680. doi: 10.1016/j.vaccine.2016.10.039. [DOI] [PubMed] [Google Scholar]
- 6.Moro P.L., Haber P., McNeil M.M. Challenges in evaluating post-licensure vaccine safety: observations from the Centers for Disease Control and Prevention. Expert Rev Vaccines. 2019;18:1091–1101. doi: 10.1080/14760584.2019.1676154. [DOI] [PubMed] [Google Scholar]
- 7.Huber F., Ehrensperger B., Hatz C., et al. Safety of live vaccines on immunosuppressive or immunomodulatory therapy—a retrospective study in three Swiss Travel Clinics. J Travel Med. 2018;25 doi: 10.1093/jtm/tax082. [DOI] [PubMed] [Google Scholar]
- 8.Shrank W.H., Patrick A.R., Alan Brookhart M. Healthy user and related biases in observational studies of preventive interventions: a primer for physicians. J Gen Intern Med. 2011;26:546–550. doi: 10.1007/s11606-010-1609-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Remschmidt C., Wichmann O., Harder T. Frequency and impact of confounding by indication and healthy vaccinee bias in observational studies assessing influenza vaccine effectiveness: a systematic review. BMC Infect Dis. 2015;15:429. doi: 10.1186/s12879-015-1154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ji J., Sundquist J., Sundquist K. Cholera vaccine use is associated with a reduced risk of death in patients with colorectal cancer: a population-based study. Gastroenterology. 2018;154:86–92.e1. doi: 10.1053/j.gastro.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Ji J., Sundquist J., Sundquist K. Association between post-diagnostic use of cholera vaccine and risk of death in prostate cancer patients. Nat Commun. 2018;9:2367. doi: 10.1038/s41467-018-04814-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng G., Sundquist J., Sundquist K., et al. Association of post-diagnostic use of cholera vaccine with survival outcome in breast cancer patients. Br J Cancer. 2021;124:506–512. doi: 10.1038/s41416-020-01108-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aaby P., Benn C.S., Flanagan K.L., et al. The non-specific and sex-differential effects of vaccines. Nat Rev Immunol. 2020;20:464–470. doi: 10.1038/s41577-020-0338-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodridge H.S., Ahmed S.S., Curtis N., et al. Harnessing the beneficial heterologous effects of vaccination. Nat Rev Immunol. 2016;16:392–400. doi: 10.1038/nri.2016.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butkeviciute E., Jones C.E., Smith S.G. Heterologous effects of infant BCG vaccination: potential mechanisms of immunity. Future Microbiol. 2018;13:1193–1208. doi: 10.2217/fmb-2018-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins J.P.T., Soares-Weiser K., López-López J.A., et al. Association of BCG, DTP, and measles containing vaccines with childhood mortality: systematic review. BMJ. 2016;355:i5170. doi: 10.1136/bmj.i5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrews N., Stowe J., Thomas S.L., et al. The risk of non-specific hospitalised infections following MMR vaccination given with and without inactivated vaccines in the second year of life. Comparative self–controlled case-series study in England. Vaccine. 2019;37:5211–5217. doi: 10.1016/j.vaccine.2019.07.059. [DOI] [PubMed] [Google Scholar]
- 18.Sørup S., Benn C.S., Poulsen A., et al. Simultaneous vaccination with MMR and DTaP-IPV-Hib and rate of hospital admissions with any infections: a nationwide register based cohort study. Vaccine. 2016;34:6172–6180. doi: 10.1016/j.vaccine.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization . 2nd ed. World Health Organisation; Geneva: 2004. ICD-10: international statistical classification of diseases and related health problems: tenth revision.https://www.who.int/standards/classifications/classification-of-diseases [Google Scholar]
- 20.Gunderson L.L., Jessup J.M., Sargent D.J., et al. Revised TN categorization for colon cancer based on national survival outcomes data. J Clin Oncol. 2010;28:264–271. doi: 10.1200/JCO.2009.24.0952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ponticelli C., Moroni G. Hydroxychloroquine in systemic lupus erythematosus (SLE) Expert Opin Drug Saf. 2017;16:411–419. doi: 10.1080/14740338.2017.1269168. [DOI] [PubMed] [Google Scholar]
- 22.R Core Team RFfSC . R Foundation for Statistical Computing; Vienna, Austria: 2019. R: a language and environment for statistical computing. [Google Scholar]
- 23.Shi T.T., Yu X.X., Yan L.J., et al. Research progress of hydroxychloroquine and autophagy inhibitors on cancer. Cancer Chemother Pharmacol. 2017;79:287–294. doi: 10.1007/s00280-016-3197-1. [DOI] [PubMed] [Google Scholar]
- 24.Lipsitch M., Tchetgen Tchetgen E., Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology. 2010;21:383–388. doi: 10.1097/EDE.0b013e3181d61eeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nørgaard M., Ehrenstein V., Vandenbroucke J.P. Confounding in observational studies based on large health care databases: problems and potential solutions - a primer for the clinician. Clin Epidemiol. 2017;9:185–193. doi: 10.2147/CLEP.S129879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson L.A., Jackson M.L., Nelson J.C., et al. Evidence of bias in estimates of influenza vaccine effectiveness in seniors. Int J Epidemiol. 2006;35:337–344. doi: 10.1093/ije/dyi274. [DOI] [PubMed] [Google Scholar]
- 27.Kandeel M., Abdelrahman A.H.M., Oh-Hashi K., et al. Repurposing of FDA-approved antivirals, antibiotics, anthelmintics, antioxidants, and cell protectives against SARS-CoV-2 papain-like protease. J Biomol Struct Dyn. 2020;39:5129–5136. doi: 10.1080/07391102.2020.1784291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aznar M.A., Molina C., Teijeira A., et al. Repurposing the yellow fever vaccine for intratumoral immunotherapy. EMBO Mol Med. 2020;12 doi: 10.15252/emmm.201910375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.