Abstract
Ovarian cancer stands as the deadliest gynecologic malignancy, responsible for nearly 65% of all gynecologic cancer-related deaths. The challenges in early detection and diagnosis, coupled with the widespread intraperitoneal spread of cancer cells and resistance to chemotherapy, contribute significantly to the high mortality rate of this disease. Due to the absence of specific symptoms and the lack of effective screening methods, most ovarian cancer cases are diagnosed at advanced stages. While chemotherapy is a common treatment, it often leads to tumor recurrence, necessitating further interventions. In recent years, antibody-drug conjugates (ADCs) have emerged as a valuable tool in targeted cancer therapy. These complex biotherapeutics combine an antibody that specifically targets tumor specific/associated antigen(s) with a high potency anti-cancer drug through a linker, offering a promising approach for ovarian cancer treatment. The identification of molecular targets in various human tumors has paved the way for the development of targeted therapies, with ADCs being at the forefront of this innovation. By delivering cytotoxic agents directly to tumors and metastatic lesions, ADCs show potential in managing chemo-resistant ovarian cancers. Mucins such as MUC16, MUC13, and MUC1 have shown significantly higher expression in ovarian tumors as compared to normal and/or benign samples, thus have become promising targets for ADC generation. While traditional markers are limited by their elevated levels in non-cancerous conditions, mucins offer a new possibility for targeted treatment in ovarian cancer. This review comprehensively described the potential of mucins for the generation of ADC therapy, highlighting their importance in the quest to improve the outcome of ovarian cancer patients.
Keywords: Ovarian cancer, Antibody drug conjugate (ADC), Mucins, MUC1, MUC13, MUC16, FRα, NaPi2B, Mesothelin
Introduction
Ovarian cancer (OC), known as the deadliest gynecologic malignancy, remains a formidable threat to women’s health worldwide, constituting a significant proportion of cancer-related fatalities [1, 2]. Often asymptomatic in its early stages, ovarian cancer tends to be diagnosed at an advanced phase, contributing to its alarming fatality rates among gynecological cancers [3]. In the United States, it stands as a major contributor to cancer-related deaths in women, underscoring the critical need for improved screening, early detection methods, and advancements in treatment modalities [4]. In addition, the Global Cancer Observatory forecasts that by the year 2040, the number of ovarian cancer cases that are diagnosed will increase by 30%, reaching 428,966, highlighting the urgency to enhance therapeutic options and personalized treatment approaches to effectively combat this complex disease [1].
Platinum-based chemotherapy and surgical interventions have been the cornerstone of ovarian cancer treatment [5]. Additionally, the combination of chemotherapy with platinum and taxane-based drugs is currently considered the gold standard for treating ovarian cancer [6]. Although most patients initially react to chemotherapy based on platinum, about 85% of them acquire resistance to the treatment and experience recurrent disease [7–9]. Ovarian cancer patients diagnosed at stages III or IV have a dismal prognosis, with a 5-year survival rate ranging from 3 to 19%. Patients with a 5-year survival rate of 40–90% are those who are recognized early; as a result, attempts are being made to develop more effective therapy approaches to cure those identified by early detection [10]. Due to the development of resistance to chemotherapy and the occurrence of harmful side effects, new therapeutic options must be considered for ovarian cancer treatment [11]. The identification of molecular targets in many human tumors has made significant strides in recent times, laying a solid foundation for the development of targeted therapies.
The development of targeted therapies, including antibody-drug conjugates (ADCs), is crucial in overcoming chemoresistance in ovarian cancer and improving survival rates for patients battling this aggressive disease [12]. The evolving landscape of molecular targeting in cancer research has opened doors to precision medicine, offering promising prospects for the development of tailored therapies that can address the molecular diversity of ovarian cancer subtypes. Strategies such as utilizing ADCs to target specific tumor antigens like MUC1, MUC13 and MUC16 may show potential in delivering potent treatment directly to cancer cells while minimizing harm to healthy tissues, paving the path for more effective, personalized, and targeted treatment approaches in the relentless fight against ovarian cancer [13, 14].
Chemoresistance in ovarian cancer: a major challenge
Chemoresistance in ovarian cancer represents a significant challenge in the treatment of this aggressive disease [11]. While chemotherapy is often the primary treatment option for ovarian cancer, the development of resistance to these drugs can lead to treatment failure and disease progression [7–9]. This resistance can be intrinsic, meaning the cancer cells are inherently resistant to the drugs, or acquired, where the cancer cells develop resistance over time. The mechanisms underlying chemoresistance in ovarian cancer are complex and multifaceted, involving changes in drug transport, metabolism, DNA repair mechanisms, and cell survival pathways [15]. Overcoming chemoresistance in ovarian cancer is crucial for improving treatment outcomes and patient survival [11]. Researchers are focusing on identifying new therapeutic strategies, such as targeted therapies and immunotherapies, to overcome resistance and improve the effectiveness of chemotherapy in patients with ovarian cancer.
Heterogeneous nature of ovarian cancer: implications for chemotherapeutic strategies
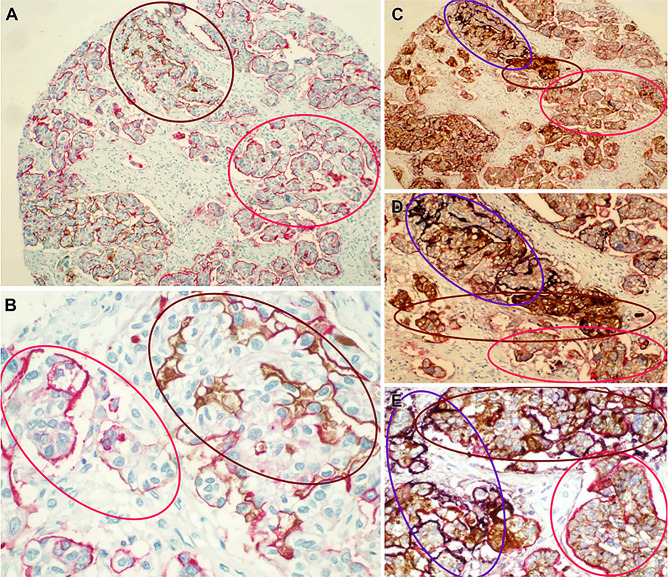
Ovarian cancer is a complex and challenging disease characterized by its heterogeneous nature [16]. This variability stems from the fact that ovarian cancer can arise from different cell types within the ovary, leading to a diverse range of tumor subtypes with distinct biological behaviors and responses to treatment. Additionally, ovarian cancer can present at different stages, with varying degrees of aggressiveness and metastatic potential [17]. The heterogeneity of ovarian cancer poses a significant clinical challenge, as it impacts the effectiveness of treatment strategies and the overall prognosis for patients. The treatment of ovarian cancer using intraperitoneal radioimmunotherapy (IRIT) targeting specific antigens has been only partially successful, mainly due to the heterogeneous expression of the targeted tumor-associated antigens by ovarian cancer cells [18]. The low response of single-antigen IRIT in ovarian tumors may be primarily caused by focal and heterogeneous expression of target antigens on individual cells, leaving many cancer cells untargeted and allowing for early disease recurrence. Designing a multi-antigen targeting strategy with antibodies against numerous tumor antigens will be a vital choice to overcome the heterogenic formation of tumor associated antigens (TAAs) by ovarian cancer cells. We have previously reported the simultaneous immunolabeling of TAG-72, MUC1, and CA125 in ovarian cancer samples [19]. Double and triple immunolabeling makes it very visible that CA125, MUC1, and TAG-72 are labeled irregularly and heterogeneously (Fig. 1). When double-staining, MUC1, +TAG-72 and MUC1 + CA125 together labeled notably greater proportion of cells than when using a single antigen (Fig. 1A and B). Furthermore, in 98% of epithelial ovarian cancer samples, triple staining achieved > 95% labeling of ovarian cancer cells (Fig. 1C–E). Multi-antigen labeling undoubtedly labeled a larger number of ovarian cancer cells, and these findings unambiguously indicate that multi-antigen targeting strategies are necessary in order to efficiently target and eradicate the majority of cells inside a malignant tumor [19]. The categorization of ovarian cancer into two groups, type I and type II, as proposed by Kurman et al., in 2008 [20]., serves as a valuable framework for understanding the disease’s varied characteristics. Type I tumors, encompassing low-grade serous, endometrioid, clear cell, and mucinous carcinomas, typically manifest at early stages, allowing for potential detection and intervention. Among the types of ovarian cancer, serous carcinoma is generally considered to have the worst prognosis. Serous carcinoma is often more aggressive non-mucinous ovarian cancer tends to spread more quickly than endometrioid and clear cell carcinomas [21]. In contrast, type II tumors exhibit high aggressiveness and typically present at advanced stages, posing significant challenges in terms of treatment and prognosis [22]. The heterogeneity of cellular composition within the peritoneum, coupled with the diverse pathological profiles of ovarian cancer subtypes, underscores the urgent need for the development of precise therapeutic strategies. These approaches should aim to optimize the bioactivity of treatment payloads, ensure targeted drug delivery to affected sites, and minimize off-target cytotoxic effects, ultimately improving outcomes for ovarian cancer patients [23]. Understanding and addressing the diverse molecular and genetic characteristics of ovarian cancer subtypes is essential for developing tailored therapies that can improve outcomes for individuals affected by this insidious disease.
Fig. 1.
Multi-antigen labeling of ovarian cancer samples. (A, B) Microarray slides of ovarian cancer tissue were prepared for double labeling with antibodies against MUC1 (Vulcan Fast Red; red) and CA125 (DAB; brown). (C–E) Samples of ovarian cancer were triple stained with TAG-72 (brown), MUC1 (red), and CA125 (purple); A portion of the content adopted with permission from ref [19]
Antibody-drug conjugates: a beacon of hope in ovarian cancer therapy
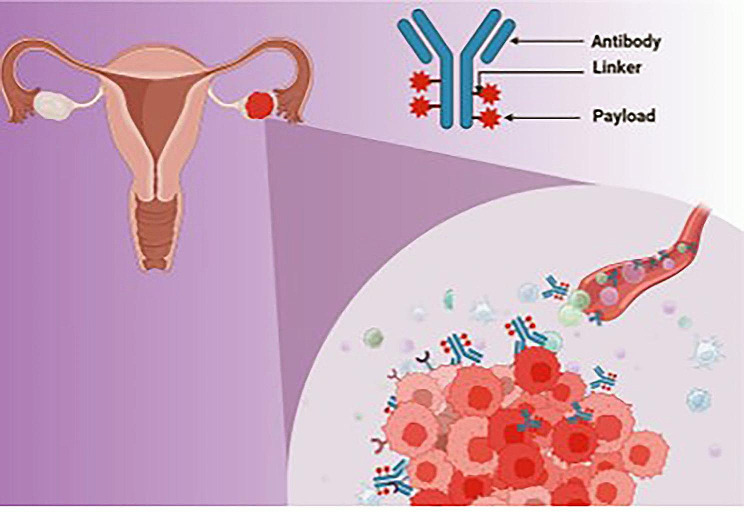
In recent years, antibody-drug conjugates have become valuable assets in the field of molecularly targeted medicine. Antibody-drug conjugates are complex biological entities that combine a surface receptor-targeting antibody with a cytotoxic chemical [24]. This reduces the adverse effects on healthy cells/tissue that expresses minimal or no levels of the targeted antigen and enables the targeted distribution and internalization of a toxic payload by tumor cells that express the antigens targeted by the ADC (Fig. 2) [25]. Additionally, the chemical structure of the linker imparts distinct qualities to the various ADCs [24, 25]. Antigen-depleting drugs featuring non-cleavable thioether linkers necessitate internalization and degradation by lysosomes to exhibit their antitumor properties without causing harm to adjacent antigen-negative cells [25]. In contrast, ADCs including cleavable linkers have the potential to discharge a portion of their lethal payload into the tumor microenvironment, resulting in the death of antigen-positive target cells as well as adjacent antigen-negative cells through the bystander effect. A multitude of tumor-antigens that are differently expressed in ovarian cancers are amenable to this innovative approach [26]. ADCs consist of three constituent elements: a synthetic chemical linker, a cytotoxic payload, and an antibody. Each component of an ADC contributes to a distinct characteristic, namely tumor selectivity, cytotoxicity, and biodistribution. The selection and formulation of individual components are critical in determining the therapeutic efficacy of an ADC.
Fig. 2.
Schematic illustration of antibody-drug conjugates in ovarian cancer treatment (created with BioRender.com)
Antibody
Selecting and designing the antibody for an ADC is vital as it acts as the carrier for delivering the cytotoxic payload to the tumor cell [27]. The antigen targeted by the ADC must be abundantly expressed on the tumor cell, minimally present in normal tissues, and located on the cell surface for specific binding. Immunoglobulins require antigen-specific design for effective binding in the Fab region. Most ADCs use IgG antibodies with variations in amino acid sequences and effector functions to enhance efficacy. Modifications like changing the electric charge or mutating cysteines can improve antibody performance, with options to create “Fc-silent” antibodies for reduced toxicity or increase effector functions for enhanced anti-tumor effects [28, 29].
Payload
Payloads in ADCs are small molecules (300–1000 Da) with IC50 values in the nanoscale to picomolar range, capable of being linked through chemical modifications. Hydrophobic payloads can cause ADC aggregation, immunogenicity, and rapid clearance [30]. Auristatins and maytansinoids are common payload classes, inhibiting tubulin assembly and exerting bystander effects. The drug antibody ratio (DAR) denotes the mean quantity of payload molecules that are securely bound to an antibody. Typically, a lowered DAR signifies a less potent ADC, whereas an increased DAR corresponds to a greater potency. A large DAR may ultimately diminish the efficacy of an antidiuretic receptor by interfering with biodistribution (e.g., accumulating in non-target organs), increasing drug clearance, and negatively altering antigen binding, depending on the payload molecule’s properties [31].
Linkers
Linkers are crucial determinants of the pharmacokinetics, pharmacodynamics, and toxicity of the entire ADC. For maximum clinical efficacy, they should be chosen in conjunction with the payload and antibody [32]. The point at which the payload is offloaded is determined by the linker design (deconjugated). The selection of coupling chemistry and linker has an impact on the DAR of an ADC. Cleavable linkers comprise motifs that are susceptible to enzymatic cleavage by proteases, hydrolysis at acidic pH, or redox processes taking place in lysosomes or early or late endosomes. Lysosomal proteolytic degradation of the antibody backbone is required for non-cleavable linkers to liberate the payload, as is the case with succinimidyl 4-N-maleimidocaproyl, which is utilized in T-DM1 (ado-trastuzumab emtansine). The electric charge and hydrophobicity/hydrophilicity of the payload may thus be influenced by linkers [33]. This has two consequences, one of which is to impair the capacity of the payload to diffuse across membranes, thereby avoiding or inducing a bystander effect on adjacent cells that do not express the antigen. As a result, intracellular concentrations of the payload may be altered, and primary or acquired drug resistance may develop. While existing linkers in clinical trials are specifically engineered to facilitate the internalization of ADCs, efforts are underway to develop new linkers that are designed to target ADCs that do not require internalization [34].
Key targets for ADCs in ovarian cancer and their significance
In ovarian cancer, the choice of payload in antibody-drug conjugates is crucial for targeting key biomarkers and pathways that play a significant role in the progression of the disease. Some of the key targets in ovarian cancer include the human epidermal growth factor receptor 2 (HER2), folate receptor alpha (FRα), and mesothelin [35–37]. HER2 is overexpressed in a subset of ovarian cancers and has been associated with more aggressive tumor behavior and poorer outcomes [38]. FRα is highly expressed in many ovarian cancer subtypes, making it an attractive target for therapy [39]. Mesothelin is another biomarker overexpressed in ovarian cancer, particularly in more aggressive subtypes like mesothelioma. Targeting these biomarkers with ADCs can potentially improve treatment outcomes by delivering cytotoxic drugs directly to tumor cells, inhibiting their growth and survival. Understanding the significance of these key targets and their role in ovarian cancer progression is essential for developing effective ADCs that can selectively kill cancer cells while sparing normal tissues. We are discussing here some of the important key targets for ovarian cancer (Table 1).
Table 1.
Ongoing preclinical and clinical trials utilizing ADC that target different antigens in the treatment of ovarian cancer
| Sr. No. | Stage | Antigen | Types of linker | Payload | ADC | Clinical trial no. | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Preclinical | TROP-2 | Cleavable 6.78 | SN-38 | Sacituzumab govitecan | [40, 41] | |
| TIM1 | Cleavable 4.5 | Monomethyl auristatin E (MMAE) | CDX-014 | [42] | |||
| PTK7 | Cleavable NR | Microtubule/Tubulin inhibitor, PF-0664178 (Aur0101) | Cofetuzumab pelidotin | [43] | |||
| 2 | Phase 1 | NOTCH-3 | Cleavable NR | Monomethyl |
PF-06650808 Praluzatamab |
NCT02129205 | [44] |
| CD166 | Cleavable 3.5 | Analogue of maytansine (DM4) | Ravtansine, CX-2009 | NCT03149549 | [45] | ||
| MUC16 | Cleavable 3.5 | MMAE | Sofituzumab vedotin | NCT01335958 | [46] | ||
| NaPi2B | Cleavable ester linker | Auristatin F-hydroxypropylamide (AF-HPA) | Upifitamab rilsodotin |
UPGRADE-A |
[47] | ||
| UPLIFT (NCT03319628) I-II | [47] | ||||||
| UP-NEXT (NCT05329545) III | [47] | ||||||
| Folate receptor α | Cathepsin cleavable linker | Eribulin | Farletuzumab ecteribulin | NCT04300556 | [47] | ||
| Folate receptor α | Protease-labile Val-Cit-PABA linker | Hemiasterlin | Luveltamab tazevibulin | NCT05200364 | [47] | ||
| 3 | Phase 2 | Tissue factor | Cleavable 3.2 | MMAE | Tisotumab vedotin | NCT03438396 | [22] |
| Mesothelin | Cleavable 3.2 | DM4 | Anetumab ravtansine | NCT03587311 | [48] | ||
| NaPi2B | Cleavable 3–4 | MMAE | Lifastuzumab vedotin | DNIB0600A | [49] | ||
| Mesothelin | Sulfo-PDB | DM4 | Anetumab ravtansine | NCT03587311 | [47, 50] | ||
| Folate receptor α | Cathepsin cleavable linker | Eribulin | Farletuzumab ecteribbulin | NCT04300556 | [47] | ||
| Folate receptor α | Sulfo-PDB cleavable linker | DM4 | Mirvetuximab soravtansine | NCT04274426 NCT05041257 | [47] | ||
| 4 | Phase 3 | Folate receptor α | Sulfo-PDB cleavable linker | DM4 | Mirvetuximab soravtansine | NCT04209855 | [51] |
| Folate receptor α | Sulfo-PDB cleavable linker | DM4 | Mirvetuximab soravtansine | NCT05445778 | [47] |
Folate receptor alpha (FRα)
Folate binding protein, alternatively referred to as glycophosphatidylinositol (GPI)-anchored transmembrane glycoprotein, is located on the cell surface. Its primary function is to enable the unidirectional transportation of folate into cells (Fig. 3). Folate is minimally expressed in healthy cells and facilitates folate uptake into cells, which is essential for DNA synthesis, cellular metabolism, and proliferation [36, 52, 53]. The up-regulation of this activity is associated with an increased demand for the enzymatic activities that take place during the one-carbon metabolism, which is a crucial aspect of the carcinogenesis process [54]. There is a minimal expression of FRα in normal adult tissues that contain polarized epithelia. These tissues include the choroid plexus, the proximal tubules of the kidney, the epithelium of the fallopian tubes, the ovaries, the uterus, and the cervix, acinar cells of the breast, and type I and type II pneumocytes of the lung [52] Ovarian, endometrial, breast, and non-small cell lung cancers are all malignancies that have abnormal or elevated expression of FRα [55, 56]. Folate is a crucial co-factor for one-carbon transfer reactions, which are necessary for the synthesis of DNA and RNA, as well as for cell growth and proliferation [55, 56]. On the other hand, it is seen to be overexpressed in as many as 90–95% of epithelial ovarian carcinomas, primarily in serous and endometrioid subtypes [39]. The expression of FRα in OC is observed to be significantly different in high-grade serous histotype, which accounts for 76% of the cases [57]. There exists a correlation between the serum levels of FRα and the levels of ovarian tissue [58]. This marker appears to increase resistance to chemotherapy and is associated with reduced progression-free survival (PFS) in localized and advanced ovarian cancer [58]. In addition to its diagnostic value [58–60], this marker also has a significant impact on the monitoring of therapy [58].
Fig. 3.
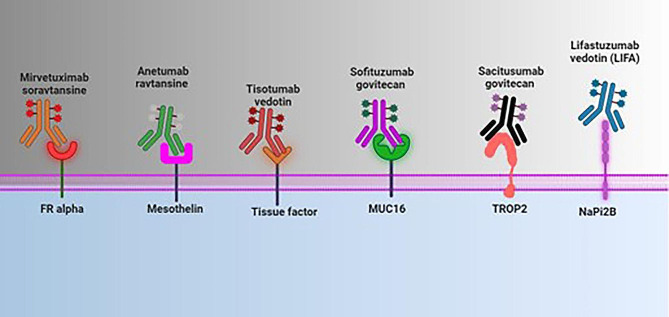
Molecular strategies and different targets for ADC in the treatment of ovarian cancer. Abbreviations: ADC: antibody drug conjugate; MUC16: Mucin 16; TROP2: trophoblast cell surface antigen 2; NaPi2B: type 2 sodium-dependent phosphate transporter (created with BioRender.com)
Mirvetuximab soravtansine, (ImmunoGen Inc., Waltham, MA, USA), is a cleavable linker-based anti-FRα antibody ADC conjugated with tubulin-targeting DM4 [61, 62]. It has shown potential activity in the treatment of epithelial ovarian cancer. The overall response rate (ORR) in a phase 1 escalation cohort of platinum-resistant ovarian cancer (n = 44) was 26%, and the median progression-free survival (mPFS) was 4.8 months. The cohort was treated with platinum radiation therapy. In order to lessen the risk of ocular toxicity, the recommended dose for phase 2 (RP2D) was determined to be 6 mg/kg administered intravenously and adjusted to the optimal body weight [61, 62]. In phase 3 of the study, Mirvetuximab sorvatansine-gynx (MIRV), was approved for the treatment of ovarian cancer patients with FRα-positive ovarian cancer. MIRV showed significantly longer progression-free survival (5.62 vs. 3.98 months, p < 0.001), higher objective response rate (42.3% vs. 15.9%, p < 0.001), and longer overall survival (median 16.46 vs. 12.75 months, p = 0.005) compared to traditional chemotherapy. MIRV also had fewer adverse events of grade 3 or higher (41.7% vs. 54.1%), fewer serious adverse events of any grade (23.9% vs. 32.9%), and fewer events leading to discontinuation (9.2% vs. 15.9%) than chemotherapy (paclitaxel, pegylated liposomal doxorubicin, or topotecan) [63, 64].
Type II sodium-dependent phosphate transporter (NaPi2B)
NaPi2B is a member of the SLC34 family of phosphate transporters, which is a multi-transmembrane type II sodium-dependent phosphate transporter [65]. Cell-surface transporter NaPi2B is a sodium-dependent protein that is routinely expressed in epithelial cells of the lungs and the small intestine [66] (Fig. 3). Serous ovarian tumor cells have been shown to exhibit a higher level of expression of this protein in comparison to non-malignant ovarian cells [67]. It is involved in the process of transporting inorganic phosphate across the cell membrane and also contributes to the maintenance of phosphate homeostasis. Under normal circumstances, it is expressed on the apical surfaces of epithelial cells, such as type II pneumocytes in the lungs, intestinal epithelium, and the epithelial lining of the uterus and fallopian tubes [68]. Eighty to ninety% of ovarian malignancies, non-squamous non-small cell lung tumors, and papillary thyroid cancers have abnormally high levels of its expression [66]. The expression of NaPi2b is found to be altered in ovarian carcinomas compared to normal tissues [69], particularly in serous and clear cell adenocarcinomas [70]. A reason for early testing in clinical trials was provided by the examination of the efficacy of anti-NaPi2b ADC utilizing tumor xenograft models when applied to ovarian and non-small cell lung malignancies [71]. It has been demonstrated that this ADC takes some time to deposit in the micro metastases of the peritoneal cavity, which indicates that it has the ability to target residual disease [68].
Lifastuzumab vedotin, also known as LIFA, is an antiNAPi2B ADC conjugated with MMAE and a protease-cleavable linker (Genetech Inc., San Francisco, Ca, USA) [47]. This study compared the efficacy of LIFA with that of the standard pegylated liposomal doxorubicin (PLD) in a phase 2 clinical trial that was conducted on 99 patients with platinum-resistant ovarian cancer who were not selected for treatment. With no variations in terms of NaPi2B expression, the median progression-free survival (PFS) was 5.3 months as opposed to 3.1 months (HR 0.71), favoring ADC. In patients who were treated with LIFA, the overall response rate (ORR) was also greater (34% versus 15%, p = 0.03). As opposed to the control group, the experimental group had a significantly higher incidence of neuropathy (11% versus 4%) [49].
Another antiNAPi2B ADC, XMT1536 (Mersana Therapeutics, Cambridge, Massachusetts, USA), possesses an auristatin payload that is conjugated through a cleavable linker. Due to the fleximer polymer linker that is present in the chemical structure, it is possible to get a larger DAR (10–12), which could be interpreted as a higher level of effectiveness. The preliminary findings from the phase 1 trial that were presented at the American Society of Clinical Oncology Congress (ASCO 2019 and ASCO 2020) demonstrated that platinum-resistant ovarian cancer patients experienced 2 CR and eleven prolonged stable disease without experiencing any significant adverse effects [72].
Mesothelin
Mesothelin is a glycoprotein that is connected to the membrane and is associated with GPI (Fig. 3). Its expression is rare in non-cancerous tissues and is restricted to normal mesothelial cells in the pericardium, peritoneum, and pleura [73]. The expression of this gene is elevated in nearly all instances of mesothelioma, ovarian malignancies, and pancreatic cancers, as well as in 50% of lung and gastric cancers, and in two-thirds of triple negative breast cancers [74]. There is a correlation between a high mesothelin expression and a worse prognosis in EOC [75]. In preclinical investigations, anetumab ravtansine (BAY 94-9343, Bayer AG, Leverkusen, Germany), an ADC containing DM4 and including a cleavable linker, shows remarkable efficacy when combined with PLD. In platinum-resistant illness, a phase 1b trial including PLD and the drug demonstrated sustained responses, with a disease control rate (DCR) of 83%, 52% PR (11/21), and 33% stable disease (7/21) [76].
Tissue factor (TF)
Tissue factor is a widely recognized extrinsic coagulation factor that exhibits abnormal expression in numerous solid tumors, such as epithelial ovarian cancer. Tisotumab vedotin (30), manufactured by Seattle Genetics Inc. in Bothell, WA, USA, and Genmab in Copenhagen, Denmark, conjugates MMAE via a protease cleavable valine-citrulline linker. The results of the phase 1 InnovaTV201 trial in patients with ovarian cancer indicate only moderate activity (ORR 13.9%) [75]. An ongoing phase 2 experiment (NCT03657043; InnovaTV208; ClinicalTrials.gov) is examining platinum-resistant ovarian cancer [75].
Mucins as therapeutic targets: harnessing antibody-drug conjugates for ovarian cancer treatment
Ovarian cancer is a challenging and often fatal gynecological malignancy that presents a pressing need for innovative therapeutic approaches. Mucins are a family of glycoproteins that play important roles in maintaining the protective barrier of epithelial cells and are involved in various cellular processes, including cell signaling, adhesion, and differentiation. In ovarian tumors, aberrant expression of mucins has been observed, which can impact tumor growth, progression, and response to therapy [4]. Antibody drug conjugates targeting specific cell surface antigens have emerged as a promising strategy for the treatment of ovarian cancer.
ADCs directed against mucin family members MUC16 and MUC1 have shown significant potential in the management of ovarian cancer. Targeting mucins with ADCs allows for specific delivery of cytotoxic agents to tumor cells with minimal toxicity to normal tissues. The combination of monoclonal antibodies that selectively bind to mucin antigens conjugated to potent cytotoxic drugs forms the basis of these ADCs. MUC16, also known as CA-125, is a well-known biomarker for ovarian cancer and is frequently overexpressed in ovarian tumors. ADCs targeting MUC16 can deliver cytotoxic agents directly to tumor cells, leading to specific tumor cell killing. MUC13 and MUC1 are also overexpressed in ovarian cancer and have been implicated in tumor progression and therapy resistance. ADCs targeting these mucins offer a targeted approach to attacking cancer cells while minimizing damage to normal cells. Preclinical studies investigating ADCs targeting MUC16 and MUC1 in ovarian cancer have demonstrated promising results. These studies have shown selective killing of tumor cells, inhibition of tumor growth, and improved survival outcomes in preclinical models. Combination therapy using ADCs targeting multiple mucin antigens has shown synergistic effects, suggesting potential benefits for ovarian cancer patients. Early-phase clinical trials evaluating the safety and efficacy of ADCs targeting MUC16 and MUC1 in ovarian cancer are ongoing. Initial results have shown encouraging antitumor activity and tolerable toxicity profiles, indicating the feasibility and potential efficacy of these targeted therapies in ovarian cancer patients. Further clinical studies are needed to optimize dosing regimens, evaluate long-term outcomes, and determine the potential for combining ADCs with other treatment modalities. The following paragraph discusses the importance of mucins at the preclinical and clinical levels in ADC development to treat the deadliest ovarian cancer.
Pre-clinical studies
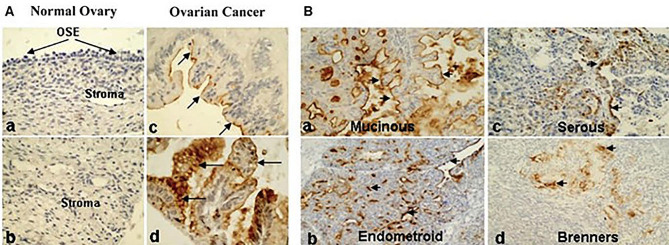
Different studies on the expression of mucins in ovarian tumors have also shown overexpression of MUC1, MUC2, MUC3, MUC4, MUC5AC and MUC13 [77–82]. In northern blot analysis a higher expression of MUC3 and MUC4 was reported in early-stage ovarian tumor samples compared to the late-stage ovarian tumor samples, and it was proposed that they provided a protective function in ovarian cancer [78]. The overexpression of MUC1 in various types and stages of ovarian tumor samples is reported in several studies [78, 79, 83]. Our laboratory has also found abnormal expression of a newly identified membrane-anchored mucin, MUC13, in ovarian cancer, which aligns with these studies [84]. Differential expression of MUC13 in ovarian cancer presents an intriguing avenue for exploring the role of this biomarker in the pathogenesis and progression of the disease. Our laboratory used immunohistochemistry to analyze the MUC13 expression pattern in tissue samples from both benign and malignant ovarian cancers [84]. Normal and benign ovarian samples did not exhibit any MUC13 expression, while samples with ovarian cancer displayed a markedly elevated MUC13 expression (Fig. 4Aa-d). In most cases, MUC13 primarily resided on the apical membrane (Fig. 4Ac), however in certain instances, it was also found in the cytoplasm (Fig. 4Ad). Further, we augmented MUC13 expression study with 10 benign/normal ovarian samples and 56 clinically confirmed cases of epithelial ovarian carcinoma (EOC). In our analysis, MUC13 overexpression was detected in 66.0% of the EOC samples (Fig. 4B). The oncogenic potential of MUC13 has been established because of the high expression of MUC13 in cancer cells as well as recent laboratory studies that imply a malignant phenotype of cell lines that have been transfected with MUC13. The up regulation of HER2, PAK1, p38, JNK signaling pathways and tumor growth in xenograft models in response to MUC13 expression further supports its role in promoting cellular growth and invasive characteristics in ovarian cancer cells [84]. Serum levels of MUC13 were found to be higher in malignant ovarian cancer compared to benign cases. Both MUC13 and CA125 were shown to have similar diagnostic performance with AUC values of 0.74 and 0.76 respectively. This further suggests that MUC13 can serve as a supplementary biomarker in detection of certain types of ovarian cancer [85]. By elucidating the molecular mechanisms underlying the dysregulation of MUC13 in ovarian cancer, researchers can uncover novel therapeutic strategies, including the development of ADCs targeting MUC13-positive tumor cells. Further exploration of the differential expression of MUC13 in ovarian cancer holds promise for advancing our understanding of the disease and identifying new opportunities for precision medicine approaches to improve patient outcomes.
Fig. 4.
MUC13 expression in ovarian cancer was evaluated by immunohistochemistry. A, TMA. For immunohistochemical analysis, TMA slides containing ovarian carcinoma were processed. Analysis of stromal tissue and OSE from a healthy ovary revealed no detectable MUC13 expression (a, b). MUC13 (arrows) was detected in ovarian cancer samples via membrane-bound (c) and cytoplasmic (d) staining. B, Clinical tissues. In a variety of EOC samples, the expression of MUC13 was observed. Membrane-bound (a, b) and cytoplasmic (c, d) immunostaining for MUC13 in EOC samples are depicted in the four representative panels. Magnification at the outset was 200x. (Adopted with permission from ref [84])
Another mucin, MUC1 has also been studied in relation to its aberrant expression and pathological functions in ovarian carcinoma. MUC1 is a transmembrane mucin that is overexpressed in ovarian cancer and has been shown to influence the metastatic ability of ovarian carcinoma [86]. Currently, it is also being explored as a target for antibody-mediated tumor therapy [87, 88]. Preclinical and clinical studies have tested mucin-based immunoconjugates for ovarian cancer treatment. Antibody-cytotoxin conjugates, such as humanized CTM01 antibody against MUC1 conjugated with calicheamicin, exhibited remarkable therapeutic efficacy, even against cisplatin-resistant ovarian tumor xenografts. Similarly, an antibody against MUC16 (3A5) conjugated with a cytotoxic drug demonstrated potential toxicity against tumor cells both in vitro and in xenograft models. These findings highlight the potential of mucin-targeting strategies for improving ovarian cancer treatment outcomes [89].
Clinical studies
It has been shown that malignant ovarian tumors often express more mucins than benign and borderline ovarian tumors. One of the surface mucin-like glycoprotein antigens is known as mucin 16 or MUC16. According to Bast et al., 1983 [90] research, CA-125 is expressed in more than 95% of all non-mucinous epithelial OC that is in the Stage III/IV stage. According to Suh et al., 2010 [91] the serum level of CA-125 is the biomarker that is utilized the most commonly for the identification of OC. The MUC16 gene is responsible for encoding the large transmembrane glycoprotein known as MUC16, which is sometimes referred to as CA125 [92]. MUC16 is a transmembrane mucin that, when cleaved extracellularly, results in the release of soluble CA125 extracellularly [93]. Low level expression of this protein is seen on epithelial surfaces, where it ordinarily performs its function as a component of a protective mucus layer [94]. These cancers pancreatic, lung, ovarian, and endometrial are characterized by an overexpression of MUC16. In addition to promoting tumor cell invasion, adhesion, and metastasis by binding of mesothelin [95, 96], it is also involved in reducing the rate of apoptosis in tumor cells [97], protecting tumor cells from immune attack [98], and preventing tumor cells from dying.
Robert C. Bast and his colleagues made the initial discovery of MUC16 to be present in epithelial ovarian cancer in the year 1981 [99]. In a wide variety of solid tumors, notably OC, this membrane-spanning mucin with a high molecular weight is overexpressed. It plays an important function in the development of tumors and in the spread of cancer [100, 101]. Early identification, monitoring therapeutic response and recurrence, and predicting survival outcomes are all areas in which MUC16/CA125 has been developed and is widely utilized in oncology [102, 103].
Another MUC16-targeting ADC explored in an early phase I trial for patients with PROC is DMUC4064A, which was manufactured by Genentech and bears the NCT02146313 designation. This cutting-edge ADC makes use of THIOMABTM technology to deliver a more consistent ratio of medication to antibody, with anti-microtubule monomethyl auristatin E serving as the payload [104]. Within the expansion cohort of this dose escalation trial, twenty patients diagnosed with PROC were administered 5.2 mg/kg of intravenous DMUC4064A every three days [76]. There were several side effects that were documented, the most prevalent of which were blurred vision (65%), fatigue (40%), nausea (40%), peripheral neuropathy (35%), keratitis (30%), diarrhea (25%), and dry eyes (25%). It is important to note that 75% of the patients experienced grade 2 and 3 ocular problems, which necessitated the use of rewetting drops, dose reductions, and the administration of steroids. Additionally, grade 2 peripheral neuropathy was observed in a few patients, which necessitated the cessation of treatment. On the basis of RECIST criteria, the overall response rate (ORR) was 45% at a dosage of 5.2 mg/kg, and the median progression-free survival (PFS) was 5.8 months [76]. According to the information that we have, there are no phase II studies that are currently being conducted with these ADCs, and it appears that their development has been halted (AdisInsight, accessed on November 27th, 2020).
DMUC5754A, also known as sofituzumab vedotin, is an anti-MUC16 ADC utilizing a humanized IgG1 conjugated to an MMAE payload via a cleavable maleimidocaproyl-valine-citrulline-p-amino-benzyloxycarbonyl linker [46]. Patients were pre-screened for MUC16 IHC expression on archival tumors or met the surrogate criteria of an elevated serum CA125 that was at least two times the upper limit of normal in a phase I trial of DMUC5754A in platinum-resistant EOC or unresectable pancreatic cancer [46]. This was done to determine whether patient candidates met the criteria. In the beginning, patients were given DMUC5754A on a dose escalation plan that was administered every three weeks. Subsequently, they were placed in a separate escalation cohort that received weekly dosing. Platinum-resistant EOC was present in 66 of the 77 patients who were treated, and 11 of them had pancreatic cancer. 44 of the EOC patients were treated on a regimen that occurred every three weeks, and the median number of previous lines of therapy that they had received was four. Twenty-two of the patients with EOC were treated in the subsequent cohort that received weekly dosage. These patients had received a median of five lines of medication prior to receiving treatment. The RP2D was calculated to be 2.4 mg/kg per three weeks, which is equivalent to 1.4 mg/kg every week. It was well tolerated, with common adverse effects of all grades including fatigue, peripheral neuropathy, nausea, vomiting, decreased appetite, and alopecia. 16% of EOC patients with evaluable tumor samples showed MUC16 IHC 2 + expression, whereas 64% had IHC 3 + expression; this is in consideration of the fact that the EOC dose expansion group was enriched for IHC 2+/3 + expression. There was one verified CR, six confirmed PRs, two unconfirmed PRs, and six with SD lasting over six months among the EOC responders [46]. According to IHC, all EOC responders showed MUC16 expression of 2 + or 3+. When assessing serologic response, a more rigorous criterion of at least 70% reduction in CA125 was applied to the CA125 response, given that DMUC5754A binds CA125. By applying this criterion, a response rate of 25% was observed, or 7 out of 29 patients. HE4 was also investigated as a potential surrogate marker for treatment response in this experiment. By establishing a response threshold of at least 40% reduction in HE4, HE4 was found to relate to radiographic response, with a HE4 response rate of 22% (5 of 23 patients) [46].
Future directions: advancements in mucins and ADC research for ovarian cancer treatment
Through continued innovation and collaboration, researchers are poised to drive forward the development of next-generation therapies that may transform the landscape of ovarian cancer care, offering new hope for patients battling this devastating disease. The potential for combining ADCs with other targeted therapies or immunotherapies further expands the possibilities for improving patient outcomes and overcoming treatment resistance. In recent years, there has been a growing interest in exploring the potential of mucins and antibody-drug conjugates (ADCs) in the treatment of ovarian cancer. Mucins, a family of glycoproteins that play a key role in cell signaling and adhesion, have been identified as promising targets for therapeutic interventions in cancer. As researchers continue to uncover the intricate mechanisms that explain the role of mucins in the progression of ovarian cancer, a clearer understanding of how these glycoproteins contribute to tumor growth and spread is becoming apparent. Advancements in mucin and ADC research have opened new avenues for personalized medicine approaches in ovarian cancer treatment. In conclusion, the future of mucins and ADC research for ovarian cancer treatment holds great promise for advancing the field of oncology.
Acknowledgements
N/A.
Author contributions
Conceived the idea: S.C.C, and S.M., Writing Original draft. preparation: S.M., and M.S; Supervision: S.C.C; Proofreading and editing: S.M, M.S., D.Z., N.B., M.M.Y., M.C.B., and S.C.C. All authors have reviewed and given their approval to the final manuscript.
Funding
This work was partially supported by a Start-up grant from the Department of Immunology and Microbiology, School of Medicine, University of Texas Rio Grande Valley, as well as NIH grants (SC1GM139727, R01 CA210192, and R01 CA206069). The authors would like to thank the CPRIT (RP210180 and RP230419) and the UT-System Start Award facilities.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, et al. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. [DOI] [PubMed] [Google Scholar]
- 3.Orr B, Edwards RP. Diagnosis and treatment of ovarian cancer. Hematol Oncol Clin North Am. 2018;32(6):943–64. [DOI] [PubMed] [Google Scholar]
- 4.Siegel RL et al. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. [DOI] [PubMed]
- 5.Lheureux S, et al. Epithelial ovarian cancer. Lancet. 2019;393(10177):1240–53. [DOI] [PubMed] [Google Scholar]
- 6.Della Pepa C, et al. Ovarian cancer standard of care: are there real alternatives? Chin J Cancer. 2015;34(1):17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foley OW, Rauh-Hain JA, del Carmen MG. Recurrent epithelial ovarian cancer: an update on treatment. Oncol (Williston Park). 2013;27(4):288–94. [PubMed] [Google Scholar]
- 8.Liu J, Jiao X, Gao Q. Neoadjuvant chemotherapy-related platinum resistance in ovarian cancer. Drug Discov Today. 2020;25(7):1232–8. [DOI] [PubMed] [Google Scholar]
- 9.Cowan, R., et al., Primary surgery or neoadjuvant chemotherapy in advanced ovarian cancer: the debate continues…. Am Soc Clin Oncol Educ Book. 2016;36:153–162. [DOI] [PubMed]
- 10.Whitwell HJ, et al. Improved early detection of ovarian cancer using longitudinal multimarker models. Br J Cancer. 2020;122(6):847–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Payandeh Z, et al. Ofatumumab monoclonal antibody affinity maturation through in silico modeling. Iran Biomed J. 2018;22(3):180–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parit S, et al. Antibody-drug conjugates: a promising breakthrough in cancer therapy. Int J Pharm. 2024;659:124211. [DOI] [PubMed] [Google Scholar]
- 13.Nicolaides NC, Kline JB, Grasso L. NAV-001, a high-efficacy antibody-drug conjugate targeting mesothelin with improved delivery of a potent payload by counteracting MUC16/CA125 inhibitory effects. PLoS One. 2023;18(5):e0285161. [DOI] [PMC free article] [PubMed]
- 14.Brassard J, et al. Antibody-drug conjugates targeting tumor-specific mucin glycoepitopes. Front Biosci (Landmark Ed). 2022;27(11):301. [DOI] [PubMed] [Google Scholar]
- 15.Li SS, Ma J, Wong AST. Chemoresistance in ovarian cancer: exploiting cancer stem cell metabolism. J Gynecol Oncol. 2018;29(2):e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vázquez-García I, et al. Ovarian cancer mutational processes drive site-specific immune evasion. Nature. 2022;612(7941):778–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng X, et al. Single-cell analyses implicate ascites in remodeling the ecosystems of primary and metastatic tumors in ovarian cancer. Nat Cancer. 2023;4(8):1138–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao X, et al. Epithelial ovarian cancer stem-like cells expressing α-gal epitopes increase the immunogenicity of tumor associated antigens. BMC Cancer. 2015;15:956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chauhan SC, et al. Combined staining of TAG-72, MUC1, and CA125 improves labeling sensitivity in ovarian cancer: antigens for multi-targeted antibody-guided therapy. J Histochem Cytochem. 2007;55(8):867–75. [DOI] [PubMed] [Google Scholar]
- 20.Kurman RJ, et al. Early detection and treatment of ovarian cancer: shifting from early stage to minimal volume of disease based on a new model of carcinogenesis. Am J Obstet Gynecol. 2008;198(4):351–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bogani G, et al. Uterine serous carcinoma. Gynecol Oncol. 2021;162(1):226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim A, et al. Therapeutic strategies in epithelial ovarian cancer. J Exp Clin Cancer Res. 2012;31(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nezhat FR, et al. New insights in the pathophysiology of ovarian cancer and implications for screening and prevention. Am J Obstet Gynecol. 2015;213(3):262–7. [DOI] [PubMed] [Google Scholar]
- 24.Nagayama A, et al. Antibody-drug conjugates for the treatment of solid tumors: clinical experience and latest developments. Target Oncol. 2017;12(6):719–39. [DOI] [PubMed] [Google Scholar]
- 25.Staudacher AH, Brown MP. Antibody drug conjugates and bystander killing: is antigen-dependent internalisation required? Br J Cancer. 2017;117(12):1736–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lawrence MS, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499(7457):214–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen B-Q, et al. Conjugation site modulates the in vivo stability and therapeutic activity of antibody-drug conjugates. Nat Biotechnol. 2012;30(2):184–9. [DOI] [PubMed] [Google Scholar]
- 28.McDonagh CF, et al. Engineered antibody–drug conjugates with defined sites and stoichiometries of drug attachment. Protein Eng Des Selection. 2006;19(7):299–307. [DOI] [PubMed] [Google Scholar]
- 29.Junutula JR, et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat Biotechnol. 2008;26(8):925–32. [DOI] [PubMed] [Google Scholar]
- 30.Wagner-Rousset E, et al. Antibody-drug conjugate model fast characterization by LC-MS following IdeS proteolytic digestion. MAbs. Taylor & Francis; 2014. [DOI] [PMC free article] [PubMed]
- 31.Sun X, et al. Effects of drug–antibody ratio on pharmacokinetics, biodistribution, efficacy, and tolerability of antibody–maytansinoid conjugates. Bioconjug Chem. 2017;28(5):1371–81. [DOI] [PubMed] [Google Scholar]
- 32.Tsuchikama K, An Z. Antibody-drug conjugates: recent advances in conjugation and linker chemistries. Protein Cell. 2018;9(1):33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beck A, et al. Strategies and challenges for the next generation of antibody–drug conjugates. Nat Rev Drug Discovery. 2017;16(5):315–37. [DOI] [PubMed] [Google Scholar]
- 34.Rossin R, et al. Chemically triggered drug release from an antibody-drug conjugate leads to potent antitumour activity in mice. Nat Commun. 2018;9(1):1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Y, et al. The application of HER2 and CD47 CAR-macrophage in ovarian cancer. J Transl Med. 2023;21(1):654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bergamini A, et al. Folate receptor alpha antagonists in preclinical and early stage clinical development for the treatment of epithelial ovarian cancer. Expert Opin Investig Drugs. 2016;25(12):1405–12. [DOI] [PubMed] [Google Scholar]
- 37.Hassan R, Bera T, Pastan I. Mesothelin: a new target for immunotherapy. Clin Cancer Res. 2004;10(12):3937–42. [DOI] [PubMed] [Google Scholar]
- 38.Harris FR, et al. Targeting HER2 in patient-derived xenograft ovarian cancer models sensitizes tumors to chemotherapy. Mol Oncol. 2019;13(2):132–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalli KR, et al. Folate receptor alpha as a tumor target in epithelial ovarian cancer. Gynecol Oncol. 2008;108(3):619–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cardillo TM, et al. Sacituzumab Govitecan (IMMU-132), an anti-trop-2/SN-38 antibody-drug conjugate: characterization and efficacy in pancreatic, gastric, and other cancers. Bioconjug Chem. 2015;26(5):919–31. [DOI] [PubMed] [Google Scholar]
- 41.Goldenberg DM, Sharkey RM. Sacituzumab Govitecan, a novel, third-generation, antibody-drug conjugate (ADC) for cancer therapy. Expert Opin Biol Ther. 2020;20(8):871–85. [DOI] [PubMed] [Google Scholar]
- 42.Thomas LJ, et al. Development of a novel antibody-drug conjugate for the potential treatment of ovarian, lung, and renal cell carcinoma expressing TIM-1. Mol Cancer Ther. 2016;15(12):2946–54. [DOI] [PubMed] [Google Scholar]
- 43.Damelin M et al. A PTK7-targeted antibody-drug conjugate reduces tumor-initiating cells and induces sustained tumor regressions. Sci Transl Med. 2017;9(372). [DOI] [PubMed]
- 44.Rosen LS, et al. A phase I, dose-escalation study of PF-06650808, an anti-Notch3 antibody-drug conjugate, in patients with breast cancer and other advanced solid tumors. Invest New Drugs. 2020;38(1):120–30. [DOI] [PubMed] [Google Scholar]
- 45.Boni V, et al. Praluzatamab Ravtansine, a CD166-targeting antibody-drug conjugate, in patients with advanced solid tumors: an open-label phase I/II trial. Clin Cancer Res. 2022;28(10):2020–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu JF, et al. Phase I study of safety and pharmacokinetics of the anti-MUC16 antibody-drug conjugate DMUC5754A in patients with platinum-resistant ovarian cancer or unresectable pancreatic cancer. Ann Oncol. 2016;27(11):2124–30. [DOI] [PubMed] [Google Scholar]
- 47.McNamara B, et al. Value of antibody drug conjugates for gynecological cancers: a modern appraisal following recent FDA approvals. Int J Womens Health. 2023;15:1353–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.El Bairi K, Jarroudi OA, Afqir S. Revisiting antibody-drug conjugates and their predictive biomarkers in platinum-resistant ovarian cancer. Semin Cancer Biol. 2021;77:42–55. [DOI] [PubMed] [Google Scholar]
- 49.Banerjee S, et al. Anti-NaPi2b antibody-drug conjugate lifastuzumab vedotin (DNIB0600A) compared with pegylated liposomal doxorubicin in patients with platinum-resistant ovarian cancer in a randomized, open-label, phase II study. Ann Oncol. 2018;29(4):917–23. [DOI] [PubMed] [Google Scholar]
- 50.Bulat IM, Hacetrean KN, Chung A, Rajagopalan JW, Xia P, Laurent C, Childs D, Santin BH. A., Phase ib study of anti-mesothelin antibody drug conjugate anetumab ravtansine in combination with pegylated liposomal doxorubicin in platinum-resistant ovarian, fallopian tube, or primary peritoneal cancer. J Clin Oncol. 2018;36.
- 51.Moore KN, et al. Mirvetuximab Soravtansine in FRα-positive, platinum-resistant ovarian cancer. N Engl J Med. 2023;389(23):2162–74. [DOI] [PubMed] [Google Scholar]
- 52.Elnakat H. Distribution, functionality and gene regulation of folate receptor isoforms: implications in targeted therapy. Adv Drug Deliv Rev. 2004;56(8):1067–84. [DOI] [PubMed] [Google Scholar]
- 53.Assaraf YG, Leamon CP, Reddy JA. The folate receptor as a rational therapeutic target for personalized cancer treatment. Drug Resist Updates. 2014;17(4–6):89–95. [DOI] [PubMed] [Google Scholar]
- 54.Cheung A, et al. Targeting folate receptor alpha for cancer treatment. Oncotarget. 2016;7(32):52553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Toffoli G, et al. Overexpression of folate binding protein in ovarian cancers. Int J Cancer. 1997;74(2):193–8. [DOI] [PubMed] [Google Scholar]
- 56.Boogerd LS, et al. Concordance of folate receptor-α expression between biopsy, primary tumor and metastasis in breast cancer and lung cancer patients. Oncotarget. 2016;7(14):17442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Köbel M, et al. Evidence for a time-dependent association between FOLR1 expression and survival from ovarian carcinoma: implications for clinical testing. An Ovarian Tumour Tissue Analysis consortium study. Br J Cancer. 2014;111(12):2297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kurosaki A, et al. Serum folate receptor alpha as a biomarker for ovarian cancer: implications for diagnosis, prognosis and predicting its local tumor expression. Int J Cancer. 2016;138(8):1994–2002. [DOI] [PubMed] [Google Scholar]
- 59.Leung F, et al. Folate-receptor 1 (FOLR1) protein is elevated in the serum of ovarian cancer patients. Clin Biochem. 2013;46(15):1462–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Farran B, et al. Serum folate receptor α (sFR) in ovarian cancer diagnosis and surveillance. Cancer Med. 2019;8(3):920–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moore KN, et al. Phase 1 dose-escalation study of mirvetuximab soravtansine (IMGN853), a folate receptor α-targeting antibody-drug conjugate, in patients with solid tumors. Cancer. 2017;123(16):3080–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moore KN, et al. Safety and activity of Mirvetuximab Soravtansine (IMGN853), a folate receptor alpha-targeting antibody-drug conjugate, in platinum-resistant ovarian, fallopian tube, or primary peritoneal cancer: a phase I expansion study. J Clin Oncol. 2017;35(10):1112–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moore KN, et al. Phase III, randomized trial of mirvetuximab soravtansine versus chemotherapy in patients with platinum-resistant ovarian cancer: primary analysis of FORWARD I. Ann Oncol. 2021;32(6):757–65. [DOI] [PubMed] [Google Scholar]
- 64.Zhu Y et al. Mirvetuximab soravtansine in platinum-resistant recurrent ovarian cancer with high folate receptor-alpha expression: a cost-effectiveness analysis. J Gynecol Oncol. 2024. [DOI] [PMC free article] [PubMed]
- 65.Nurgalieva AK, et al. Sodium-dependent phosphate transporter NaPi2b as a potential predictive marker for targeted therapy of ovarian cancer. Biochem Biophys Rep. 2021;28:101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Feild JA, et al. Cloning and functional characterization of a sodium-dependent phosphate transporter expressed in human lung and small intestine. Biochem Biophys Res Commun. 1999;258(3):578–82. [DOI] [PubMed] [Google Scholar]
- 67.Levan K, et al. Immunohistochemical evaluation of epithelial ovarian carcinomas identifies three different expression patterns of the MX35 antigen, NaPi2b. BMC Cancer. 2017;17(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Finstad CL, et al. Distribution of radiolabeled monoclonal antibody MX35 F (ab’) 2 in tissue samples by storage phosphor screen image analysis: evaluation of antibody localization to micrometastatic disease in epithelial ovarian cancer. Clin cancer Research: Official J Am Association Cancer Res. 1997;3(8):1433–42. [PubMed] [Google Scholar]
- 69.Kiyamova R et al. Immunohistochemical analysis of NaPi2b protein (MX35 antigen) expression and subcellular localization in human normal and cancer tissues. Exp Oncol. 2011. [PubMed]
- 70.Soares IC, et al. In silico analysis and immunohistochemical characterization of NaPi2b protein expression in ovarian carcinoma with monoclonal antibody Mx35. Appl Immunohistochem Mol Morphol. 2012;20(2):165–72. [DOI] [PubMed]
- 71.Lin K, et al. Preclinical development of an anti-NaPi2b (SLC34A2) antibody–drug conjugate as a therapeutic for non–small cell lung and ovarian cancers. Clin Cancer Res. 2015;21(22):5139–50. [DOI] [PubMed] [Google Scholar]
- 72.Tolcher AW et al. Phase 1 dose escalation study of XMT-1536, a novel NaPi2b-targeting antibody-drug conjugate (ADC), in patients (pts) with solid tumors likely to express NaPi2b. American Society of Clinical Oncology. 2019.
- 73.Hassan R, Bera T, Pastan I. Mesothelin: a new target for immunotherapy. Clin Cancer Res. 2004;10(12 Pt 1):3937–42. [DOI] [PubMed] [Google Scholar]
- 74.Hassan R, et al. Localization of mesothelin in epithelial ovarian cancer. Appl Immunohistochem Mol Morphol. 2005;13(3):243–7. [DOI] [PubMed] [Google Scholar]
- 75.Hanaoka T, et al. Correlation between tumor mesothelin expression and serum mesothelin in patients with epithelial ovarian carcinoma: a potential noninvasive biomarker for mesothelin-targeted therapy. Mol Diagn Ther. 2017;21(2):187–98. [DOI] [PubMed] [Google Scholar]
- 76.Bulat I, et al. Phase ib study of anti-mesothelin antibody drug conjugate anetumab ravtansine in combination with pegylated liposomal doxorubicin in platinum-resistant ovarian, fallopian tube, or primary peritoneal cancer. American Society of Clinical Oncology. 2018.
- 77.Auersperg N, et al. Ovarian surface epithelium: biology, endocrinology, and pathology. Endocr Rev. 2001;22(2):255–88. [DOI] [PubMed] [Google Scholar]
- 78.Giuntoli RL 2, et al. Mucin gene expression in ovarian cancers. Cancer Res. 1998;58(23):5546–50. [PubMed] [Google Scholar]
- 79.Feng H, et al. Expression of MUC1 and MUC2 mucin gene products in human ovarian carcinomas. Jpn J Clin Oncol. 2002;32(12):525–9. [DOI] [PubMed] [Google Scholar]
- 80.Hanski C, et al. Overexpression or ectopic expression of MUC2 is the common property of mucinous carcinomas of the colon, pancreas, breast, and ovary. J Pathol. 1997;182(4):385–91. [DOI] [PubMed] [Google Scholar]
- 81.Chauhan SC, et al. Aberrant expression of MUC4 in ovarian carcinoma: diagnostic significance alone and in combination with MUC1 and MUC16 (CA125). Mod Pathol. 2006;19(10):1386–94. [DOI] [PubMed] [Google Scholar]
- 82.Chauhan SC, Kumar D, Jaggi M. Mucins in ovarian cancer diagnosis and therapy. J Ovarian Res. 2009;2:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dong Y, et al. Expression of MUC1 and MUC2 mucins in epithelial ovarian tumours. J Pathol. 1997;183(3):311–7. [DOI] [PubMed] [Google Scholar]
- 84.Chauhan SC, et al. Expression and functions of transmembrane mucin MUC13 in ovarian cancer. Cancer Res. 2009;69(3):765–74. [DOI] [PubMed] [Google Scholar]
- 85.Wen XT et al. Diagnostic efficacy of combining diffusion-weighted magnetic resonance imaging with serum mucin 1, mucin 13, and Mucin 16 in distinguishing borderline from malignant epithelial ovarian tumors. Asia Pac J Clin Oncol. 2024. [DOI] [PubMed]
- 86.Dong Y, et al. Expression of MUC1 and MUC2 mucins in epithelial ovarian tumours. J Pathology: J Pathological Soc Great Br Irel. 1997;183(3):311–7. [DOI] [PubMed] [Google Scholar]
- 87.Hamann PR, et al. A calicheamicin conjugate with a fully humanized anti-MUC1 antibody shows potent antitumor effects in breast and ovarian tumor xenografts. Bioconjug Chem. 2005;16(2):354–60. [DOI] [PubMed] [Google Scholar]
- 88.Kong B, et al. Efficacy of lentivirus-mediated and MUC1 antibody-targeted VP22-TK/GCV suicide gene therapy for ovarian cancer. Vivo (Athens Greece). 2003;17(2):153–6. [PubMed] [Google Scholar]
- 89.Singh AP, et al. Clinical potential of mucins in diagnosis, prognosis, and therapy of ovarian cancer. Lancet Oncol. 2008;9(11):1076–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bast RC Jr., et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med. 1983;309(15):883–7. [DOI] [PubMed] [Google Scholar]
- 91.Suh KS, et al. Ovarian cancer biomarkers for molecular biosensors and translational medicine. Expert Rev Mol Diagn. 2010;10(8):1069–83. [DOI] [PubMed] [Google Scholar]
- 92.Yin BW, Dnistrian A, Lloyd KO. Ovarian cancer antigen CA125 is encoded by the MUC16 mucin gene. Int J Cancer. 2002;98(5):737–40. [DOI] [PubMed] [Google Scholar]
- 93.Yin BW, Lloyd KO. Molecular cloning of the CA125 ovarian cancer antigen: identification as a new mucin, MUC16. J Biol Chem. 2001;276(29):27371–5. [DOI] [PubMed] [Google Scholar]
- 94.Kufe DW. Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer. 2009;9(12):874–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gubbels JA, et al. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol Cancer. 2006;5(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen SH, et al. Mesothelin binding to CA125/MUC16 promotes pancreatic cancer cell motility and invasion via MMP-7 activation. Sci Rep. 2013;3:1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Boivin M, et al. CA125 (MUC16) tumor antigen selectively modulates the sensitivity of ovarian cancer cells to genotoxic drug-induced apoptosis. Gynecol Oncol. 2009;115(3):407–13. [DOI] [PubMed] [Google Scholar]
- 98.Gubbels JA, et al. MUC16 provides immune protection by inhibiting synapse formation between NK and ovarian tumor cells. Mol Cancer. 2010;9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bast RC Jr., et al. Reactivity of a monoclonal antibody with human ovarian carcinoma. J Clin Invest. 1981;68(5):1331–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Das S, Batra SK. Understanding the unique attributes of MUC16 (CA125): potential implications in targeted therapy. Cancer Res. 2015;75(22):4669–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Felder M, et al. MUC16 (CA125): tumor biomarker to cancer therapy, a work in progress. Mol Cancer. 2014;13:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.El Bairi K, et al. Emerging diagnostic, prognostic and therapeutic biomarkers for ovarian cancer. Cell Oncol (Dordr). 2017;40(2):105–18. [DOI] [PubMed] [Google Scholar]
- 103.El Bairi K, et al. Prediction of therapy response in ovarian cancer: where are we now? Crit Rev Clin Lab Sci. 2017;54(4):233–66. [DOI] [PubMed] [Google Scholar]
- 104.Chu AJ. Tissue factor, blood coagulation, and beyond: an overview. Int J Inflam. 2011;2011:367284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.