Abstract
Background
Chronic nonspecific cheilitis is a complex condition characterized by persistent lip peeling and discomfort. This case report explores the clinical progression of a patient with history of tongue squamous cell carcinoma and subsequent Tislelizumab treatment, presenting with persistent lip peeling.
Case presentation
A patient with a history of tongue squamous cell carcinoma (T2N0M0), treated with chemotherapy, surgery, and Tislelizumab, presented with six months of persistent lip peeling. Clinical examination revealed distinct features of chronic nonspecific cheilitis with infectious angular cheilitis (Oral Candidiasis). A tailored treatment plan, emphasizing oral hygiene practices and local treatments with Sodium Bicarbonate, Tacrolimus ointment, and Chlortetracycline ointment. Follow-up visits demonstrated sustained improvement, highlighting the significance of individualized approaches.
Conclusions
This case underscores the importance of recognizing and managing oral manifestations in patients with a history of cancer and immunotherapy. The patient’s response to treatment suggests that a multifaceted approach, combining local therapy with lifestyle modifications, can be effective in managing chronic nonspecific cheilitis associated with immunotherapy. Routine follow-up appointments, guided by personalized medicine principles, contribute to sustained patient well-being.
Keywords: Chronic nonspecific cheilitis, Tislelizumab, Immunotherapy, Oral manifestations, Multidisciplinary approach
Background
Chronic nonspecific cheilitis is an inflammatory condition of the lips that manifests as persistent peeling, erythema, and discomfort, significantly impacting the patient’s quality of life [1]. The condition is often idiopathic, with a complex etiology that includes environmental exposures, microtrauma from lip-licking or lip-picking behaviors, and immunological dysregulation [2]. Although prevalent, particularly among certain demographics, the precise epidemiology of chronic nonspecific cheilitis remains to be fully elucidated [3].
The clinical presentation of cheilitis is characterized by dry, scaly, and crusted lips, which can evolve into fissures and secondary bacterial or fungal infections [4]. The management of cheilitis typically integrates emollients, protective barrier creams, and behavioral modifications to curtail lip manipulation, with a crucial focus on the exclusion of causative factors [1, 4].
The management of cheilitis, while traditionally focused on symptomatic relief through emollients and barrier protection, is further complicated in the context of immunotherapy. Immunotherapy, particularly with immune checkpoint inhibitors, has transformed cancer treatment paradigms by enhancing the immune system’s ability to target cancer cells [5]. Tislelizumab, a programmed death-1 (PD-1) inhibitor, has demonstrated efficacy in various malignancies, including squamous cell carcinoma [6]. The rationale for its use in this case was predicated on its established benefits in patients who have finished standard therapy. However, the immunomodulatory effects of these agents may lead to immune-related adverse events (irAEs), affecting various organ systems [7, 8]. The association between immunotherapy and oral manifestations, while recognized, remains an evolving field of study. Previous reports have highlighted conditions such as erythema and erosion of the gums and buccal mucosa [9], but the specific manifestation of chronic nonspecific cheilitis following Tislelizumab administration is less explored.
To date, there is no internationally accepted classification of cheilitis. Scholars, including Liborija Lugović-Mihić and Amanda Oakley, have proposed classification systems, each with its own merits and drawbacks [3]. In Lugović-Mihić’s classification, possible diagnoses for this case include drug-related reversible cheilitis and cheilitis related to specific diseases/systemic diseases [3]. However, since the patient’s symptoms persisted after discontinuing PD-1 drug therapy, it is challenging to classify this case as drug-related cheilitis. Considering the patient’s history of tongue squamous cell carcinoma and PD-1 immunotherapy, it aligns with the category of cheilitis associated with specific diseases/systemic diseases in Lugović-Mihić’s classification. In Oakley’s classification, a category encompasses cheilitis caused by allergies, toxins, drugs, and trauma, with drug-induced cheilitis often linked to isotretinoin use [1, 10].
The potential exacerbation of chronic nonspecific cheilitis by Tislelizumab, as observed in this case, underscores the need for a deeper understanding of the interplay between immunotherapy and oral health. This case report contributes to the growing body of literature on oral complications associated with immunotherapy. By examining the temporal relationship between Tislelizumab administration and the onset of cheilitis, this report provides clinicians with a nuanced perspective on managing patients with a history of cancer and immunotherapy. The implications extend beyond this individual case, informing the development of clinical guidelines for the prevention, recognition, and treatment of oral irAEs in the context of cancer immunotherapy. Although the cancer therapy pharmaceutical has been discontinued, treatment for oral cancer remains paramount, while nonspecific cheilitis represents a less serious health issue. Clinicians must prioritize treatment strategies based on the most critical factors for the patient’s overall health.
Case presentation
A 36-year-old female presented to our clinic with a chief complaint of persistent lip peeling spanning six months from April 2023. Her medical history revealed a prior diagnosis of Stage II tongue squamous cell carcinoma (T2N0M0), for which she underwent chemotherapy and surgery between August 2022 and February 2023 in another Stomatological hospital. Subsequently, she completed three cycles of Tislelizumab (200 mg) treatment from April to June 2023. Notably, following the initial dose of Tislelizumab, the patient experienced the first onset of lip peeling and pain, which progressively worsened with subsequent doses and ameliorated upon discontinuation. Despite the cessation of Tislelizumab, the patient continued to experience these symptoms for over four months post-treatment cessation, prompting her presentation to our hospital in November 2023. The patient admitted to a habit of peeling off the detached skin. She refuted any history of similar symptoms, usage of systemic medications, dental restorations, history of radiotherapy, smoking, and tobacco consumption. No other allergies or relevant medical history were reported. The patient underwent pathological examination at an external institution in August 2022, revealing grade 1 (WHO grading, well-differentiated) tongue squamous cell carcinoma (T2N0M0). Concurrently, blood tests, liver function assessments, and thyroid examinations exhibited normal results.
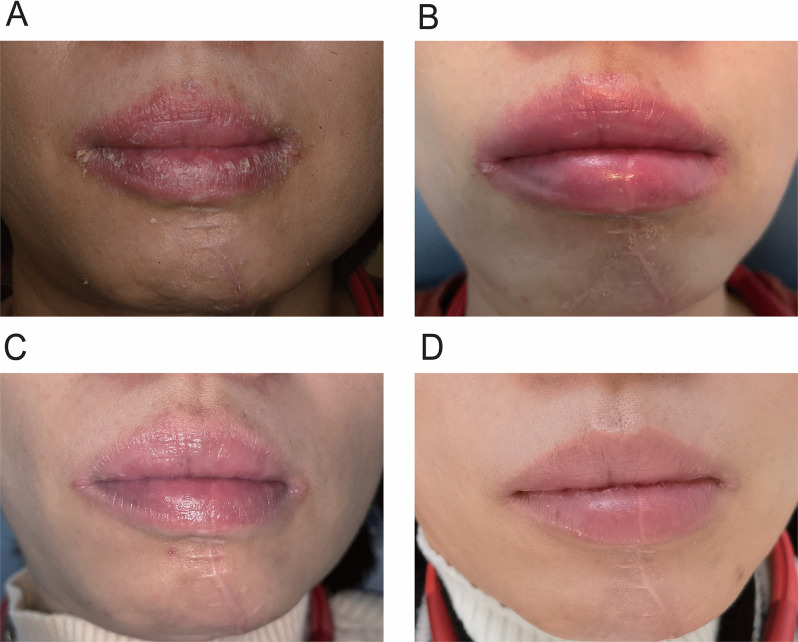
Upon examination, the patient’s upper and lower lip vermilion borders were indistinct, and both the lips and perioral skin exhibited signs of hyperemia. The lip surface was dry, with adherent yellowish crusts that could be peeled off. These findings are consistent with cheilitis. Additionally, the oral commissures manifested maceration, accompanied by wrinkles, erythema, and swelling, along with the formation of yellowish crusts, eliciting discomfort. These findings are indicative of angular cheilitis. A longitudinal surgical incision approximately 8 cm in length is observed in the lower lip and jaw area (Fig. 1A). Extraoral examination reveals no abnormalities.
Fig. 1.
Clinical manifestations, treatment process, and effects of the presented case. (A) Initial Presentation: The lip surface was dry with adherent yellowish crusts. The oral commissures showed maceration, wrinkles, erythema, swelling, and yellowish crusts, causing discomfort. (B) Local Treatment: Application of gauze with 2.5% Sodium Bicarbonate as a lip compress, once to twice daily, for 20 min each time. (C) Two-week Follow-up: The patient reported improvement in peeling symptoms. Significant enhancements in lip appearance were observed, with mild congestion on the interior vermilion and a damp, fissured condition at the left oral commissure. No swelling or tenderness was noted upon palpation. (D) One-month Follow-up: Slight congestion with a reddish hue was observed on the lower lip. Persistent yellowish scaly patches were removable, exposing normal mucosa. The left oral commissure remained damp with slight fissuring and mild congestion, without swelling or pain upon palpation
The clinical diagnosis established was chronic nonspecific cheilitis with concurrent infectious angular cheilitis based on the patient’s medical history and examination. To further elucidate the condition, a differential diagnosis was considered, which included other causes of cheilitis such as allergic reactions, contact dermatitis, actinic cheilitis, and nutritional deficiencies. However, based on the patient’s history of Tislelizumab treatment and the absence of other contributing factors, the diagnosis of Tislelizumab-associated cheilitis was deemed most probable. This conclusion was supported by the temporal association with the administration of the drug and the resolution of symptoms following its discontinuation.
In the clinical management of the patient, emphasis was placed on rigorous oral hygiene practices and recommendations were provided for adequate rest and avoidance of sun exposure. Local treatment involved the application of gauze with 2.5% Sodium Bicarbonate as a lip compress, once to twice daily, with each application lasting 10 min (Fig. 1B). Additionally, 0.03% Tacrolimus ointment was applied daily, and Chlortetracycline ointment was applied topically three times a day to treat the potential infection.
The patient returned to the clinic after a two-week treatment period, reporting self-perceived improvement in peeling symptoms. Specialized examination indicated significant enhancements in lip appearance. However, residual mild congestion on the interior vermilion and a damp, fissured condition at the left oral commissure were noted. Palpation of the lips and perioral region revealed no signs of swelling or tenderness (Fig. 1C). A daily application of Chlortetracycline eye ointment at 0.1 g was recommended.
The second follow-up visit occurred after another one-month treatment. Examination of the lower lip revealed a slight congestion with a reddish hue, and persistent yellowish scaly patches that were removable, exposing a normal mucosal base. The left oral commissure displayed characteristics of dampness, slight fissuring, and mild congestion. Palpation indicated an absence of swelling or pain (Fig. 1D). The patient was diagnosed with chronic nonspecific cheilitis. The clinical management plan included oral mucosal health guidance, encompassing recommendations for maintaining optimal oral hygiene. The treatment plan involved Sodium Bicarbonate solution as a lip compress for twice daily, emphasis on rest, avoidance of sun exposure, and adoption of a healthy diet. No recurrence was observed till the manuscript submission.
We followed the guidelines of the Helsinki Declaration in this study. The patient has been informed about her oral condition, treatment methods, costs, duration, potential complications, and prognosis. She had provided consent for all treatment.
Discussion and conclusions
Oral cancer, particularly tongue squamous cell carcinoma, is staged using the TNM classification system, which helps determine the extent of cancer spread. In this case, the patient was diagnosed with T2N0M0 tongue squamous cell carcinoma, indicating a tumor size of 2–4 cm without regional lymph node involvement or distant metastasis [11]. Treatment modalities for oral cancer typically include surgery, radiation, and chemotherapy. Recently, immunotherapy has become a crucial component, particularly using immune checkpoint inhibitors like PD-1 monoclonal antibodies.
PD-1 serves as an immunosuppressive molecule predominantly located on the surface of T-cell membranes [12]. Its ligand, programmed death protein ligand 1 (PD-L1), is expressed on various cell membranes within the human body, providing a mechanism for immune system evasion [13]. Within the tumor microenvironment, PD-L1 is expressed on the cell membranes of tumor cells. This expression leads to the binding of PD-L1 to PD-1 on the surface of T cells, resulting in the inhibition of T cell functions such as proliferation, activation, and cytokine production [14]. Ultimately, these inhibitory effects contribute to the suppression of the anti-tumor immune response [15].
Tislelizumab, a PD-1 monoclonal antibody, plays a crucial role in activating T cells to target and eliminate cancer cells [16]. This activation is achieved through the specific binding of Tislelizumab to the PD1 receptor on the surface of T cells, leading to robust anti-tumor effects [17]. This immunotherapy is indicated for various malignancies, including advanced or metastatic cancers, where conventional treatments have failed. However, it is noteworthy that the administration of Tislelizumab is associated with a spectrum of irAEs during treatment, including oral side effects.
In this case, the patient’s medical history of T2N0M0 tongue cancer treated with chemotherapy, surgery, and subsequent Tislelizumab therapy is crucial. Following the initiation of Tislelizumab, the patient developed chronic nonspecific cheilitis with infectious angular cheilitis. The onset of lip peeling and pain after starting Tislelizumab, with symptoms worsening and then improving upon discontinuation, highlights the potential immunological mechanisms at play. The PD-1/PD-L1 pathway disruption by Tislelizumab likely led to localized inflammatory responses in the oral mucosa, manifesting as cheilitis.
Previous reports have highlighted conditions such as erythema and erosion of the gums and buccal mucosa following the administration of Tislelizumab [9]. However, the specific manifestation of chronic nonspecific cheilitis in association with Tislelizumab remains less explored. These adverse effects are primarily due to the disruption of the PD-1/PD-L1 immune checkpoint pathway, leading to an overactive immune response and localized inflammation in the oral mucosa. However, the literature does not extensively cover chronic nonspecific cheilitis as a specific adverse effect of Tislelizumab.
The manifestation of cheilitis in this case suggests a potentially unique or underreported side effect of Tislelizumab. The patient’s symptoms align with the inflammatory responses observed with other oral irAEs but present in a different anatomical location and with distinct clinical features. This case highlights the need for heightened awareness and further investigation into the full spectrum of oral complications that may arise from Tislelizumab therapy.
The treatment of nonspecific cheilitis typically involves a multifaceted approach, focusing on reducing inflammation, preventing secondary infections, and maintaining optimal oral hygiene. Standard treatments include the application of topical corticosteroids to alleviate inflammation, antimicrobial ointments to prevent or treat infections, and emollients to maintain moisture and protect the lips [1]. In some cases, immunomodulatory agents such as Tacrolimus ointment may be used to suppress local immune responses [18].
In the present case, the treatment regimen demonstrated positive outcomes, with the patient reporting significant improvement in symptoms after two weeks. The follow-up visits corroborated these findings, showing reduced congestion, diminished crusting, and overall improvement in the appearance of the lips. The absence of swelling or tenderness upon palpation further indicated a favorable response to the treatment regimen.
Comparatively, the literature reports varying success rates with different treatment modalities for nonspecific cheilitis. For instance, studies have shown that topical corticosteroids and antimicrobial treatments are effective in managing symptoms, though recurrence is common if the underlying cause is not addressed [19]. The use of Tacrolimus ointment has also been documented to provide relief, particularly in cases resistant to conventional therapies. However, side effects such as burning sensations and local irritation can limit its use [20].
In this case, the combination of Sodium Bicarbonate, Tacrolimus, and Chlortetracycline proved effective, aligning with the positive outcomes reported in the literature. Sodium Bicarbonate likely contributed to creating an alkaline environment, preventing fungal infections and aiding in the exfoliation of lip crusts. Tacrolimus provided immunosuppressive effects, reducing inflammation and symptom severity, while Chlortetracycline treated the infection. The absence of swelling or tenderness upon palpation, despite the residual mild congestion, suggests a favorable response to the treatment regimen.
Notably, the patient’s compliance with the prescribed treatment and the absence of significant adverse events contribute to the overall success of the management. The detailed oral mucosal health guidance, including lifestyle recommendations and the patient’s commitment to maintaining optimal oral hygiene, aligns with the holistic approach essential in managing chronic nonspecific cheilitis.
Understanding the immunological intricacies of the patient’s response to Tislelizumab and its role in lip peeling symptoms is crucial. Tislelizumab initiation and subsequent exacerbation of lip symptoms suggest an irAE, emphasizing the need for careful management. The temporal correlation between Tislelizumab and lip symptoms, which improved upon discontinuation, highlights the importance of monitoring and adjusting treatment. The patient’s history of tongue squamous cell carcinoma adds complexity, necessitating a multidisciplinary approach. Routine follow-up is essential for monitoring response and adjusting treatment, ensuring optimal oral hygiene and preventing complications. The patient’s commitment to lifestyle recommendations fosters long-term oral health.
Looking ahead, the patient will be closely monitored for sustained treatment response and any potential complications. This vigilance is particularly crucial given the nuanced nature of the case, wherein the interplay of cancer history, immunotherapy, and chronic nonspecific cheilitis necessitates ongoing attention. The iterative nature of the follow-up appointments allows for real-time adjustments to the treatment plan based on the patient’s evolving clinical status.
In conclusion, this case highlights the need for a comprehensive, multidisciplinary approach to managing oral manifestations in cancer patients undergoing immunotherapy. Routine follow-ups and personalized medicine are essential in addressing immunotherapy-induced adverse events and the lingering effects of cancer treatments, contributing valuable insights to optimal patient care.
Acknowledgements
We appreciate the patient’s authorization for the use of clinical data.
Abbreviations
- irAEs
immune-related adverse events
- PD-1
programmed death protein 1
- PD-L1
programmed death protein ligand 1
Author contributions
Haiyue Yu and Liyi Jiang contributed to the conception of the study and drafting of the manuscript. Haiyue Yu and Qiao Ruan contributed to the oral examinations. All clinical treatments were performed by Liyi Jiang. All authors have read and approved the final manuscript.
Funding
This research was supported by the Science Research Cultivation Program of Stomatological Hospital, Southern Medical University (Grant no. PY2021026).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The patient provided informed consent for clinical management in this case. Additionally, written consent for publication of their details was obtained from the patient and complied with hospital regulations.
Consent for publication
Written informed consent was obtained from the patient to publish all clinical data and accompanying images. Additionally, written consent was obtained from the study participant to publish this information.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gonzaga AKG, de Bezerra O, Cavalcante HI, Santana IL, de Oliveira T, de Medeiros PT. An update about cheilitis. J Oral Maxillofacial Surg Med Pathol. 2021;33(5):555–60. 10.1016/j.ajoms.2021.02.001 [DOI] [Google Scholar]
- 2.Lugovic-Mihic L, Blagec T, Japundzic I, Skroza N, Delas Adzajic M, Mravak-Stipetic M. Diagnostic management of cheilitis. An approach based on a recent proposal for cheilitis classification. ACTA DERMATOVENEROLOGICA ALPINA PANONICA ET ADRIATICA. 2020;29(2):67–72. [PubMed] [Google Scholar]
- 3.Lugović-Mihić L, Pilipović K, Crnarić I, Šitum M, Duvančić T. Differential diagnosis of cheilitis–how to classify cheilitis? Acta Clin Croatica. 2018;57(2):342–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blagec T, Glavina A, Špiljak B, Bešlić I, Bulat V, Lugović-Mihić L. Cheilitis: a cross‐sectional study—multiple factors involved in the aetiology and clinical features. Oral Dis. 2023;29(8):3360–71. 10.1111/odi.14359 [DOI] [PubMed] [Google Scholar]
- 5.Farkona S, Diamandis EP, Blasutig IM. Cancer immunotherapy: the beginning of the end of cancer? BMC Med. 2016;14:73. 10.1186/s12916-016-0623-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee A, Keam SJ, Tislelizumab. First Approval Drugs. 2020;80(6):617–24. [DOI] [PubMed] [Google Scholar]
- 7.Li L, Lou A, Yu J. Immune checkpoint inhibitor-related pneumonitis induced by camrelizumab: a case report and review of literature. Ann Palliat Med. 2021;10(7):8460–6. 10.21037/apm-21-23 [DOI] [PubMed] [Google Scholar]
- 8.Zhou Q, Qin Z, Yan P, Wang Q, Qu J, Chen Y. Immune-related adverse events with severe pain and ureteral expansion as the main manifestations: a case report of tislelizumab-induced ureteritis/cystitis and review of the literature. Front Immunol. 2023;14:1226993. 10.3389/fimmu.2023.1226993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo Q, Jin L, Zhang T, Gao R, Zou K, Fu M et al. Literature analysis of cutaneous adverse reactions induced by tislelizumab. Cutaneous and Ocular Toxicology.1–6. [DOI] [PubMed]
- 10.Hon A, Oakley A. DermNet New Zealand.
- 11.Almangush A, Makitie AA, Triantafyllou A, de Bree R, Strojan P, Rinaldo A, et al. Staging and grading of oral squamous cell carcinoma: an update. Oral Oncol. 2020;107:104799. 10.1016/j.oraloncology.2020.104799 [DOI] [PubMed] [Google Scholar]
- 12.Liu T, Zhou Z, Zhang M, Lang P, Li J, Liu Z, et al. Cuproptosis-immunotherapy using PD-1 overexpressing T cell membrane-coated nanosheets efficiently treats tumor. J Controlled Release. 2023;362:502–12. 10.1016/j.jconrel.2023.08.055 [DOI] [PubMed] [Google Scholar]
- 13.Han Y, Liu D, Li L. PD-1/PD-L1 pathway: current researches in cancer. Am J cancer Res. 2020;10(3):727. [PMC free article] [PubMed] [Google Scholar]
- 14.Lotfinejad P, Kazemi T, Mokhtarzadeh A, Shanehbandi D, Niaragh FJ, Safaei S, et al. PD-1/PD-L1 axis importance and tumor microenvironment immune cells. Life Sci. 2020;259:118297. 10.1016/j.lfs.2020.118297 [DOI] [PubMed] [Google Scholar]
- 15.Tan CL, Kuchroo JR, Sage PT, Liang D, Francisco LM, Buck J et al. PD-1 restraint of regulatory T cell suppressive activity is critical for immune tolerance. J Exp Med. 2021;218(1). [DOI] [PMC free article] [PubMed]
- 16.Ye D, Desai J, Shi J, Liu S-YM, Shen W, Liu T, et al. Co-enrichment of CD8-positive T cells and macrophages is associated with clinical benefit of tislelizumab in solid tumors. Biomark Res. 2023;11(1):1–12. 10.1186/s40364-023-00465-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L, Geng Z, Hao B, Geng Q. Tislelizumab: a modified anti-tumor programmed death receptor 1 antibody. Cancer Control. 2022;29:10732748221111296. 10.1177/10732748221111296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gagari E. Cheilitis and oral disease. European handbook of dermatological treatments. Springer; 2023. pp. 137–45.
- 19.Mandasari M, Astuti AK, Rahmayanti F. A case of inconspicuous recurrent herpes labialis mimicking unilateral angular cheilitis. J Dentistry Indonesia. 2018;25(3):171–4. 10.14693/jdi.v25i3.1255 [DOI] [Google Scholar]
- 20.Liu J, Shi L, Wang X, Wu F, Hu M, He J, et al. Tacrolimus 0.03% ointment treatment in exfoliative cheilitis: a randomised controlled clinical trial and monitoring blood concentration. J Oral Pathol Med. 2021;50(2):251–9. 10.1111/jop.13142 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.