Abstract
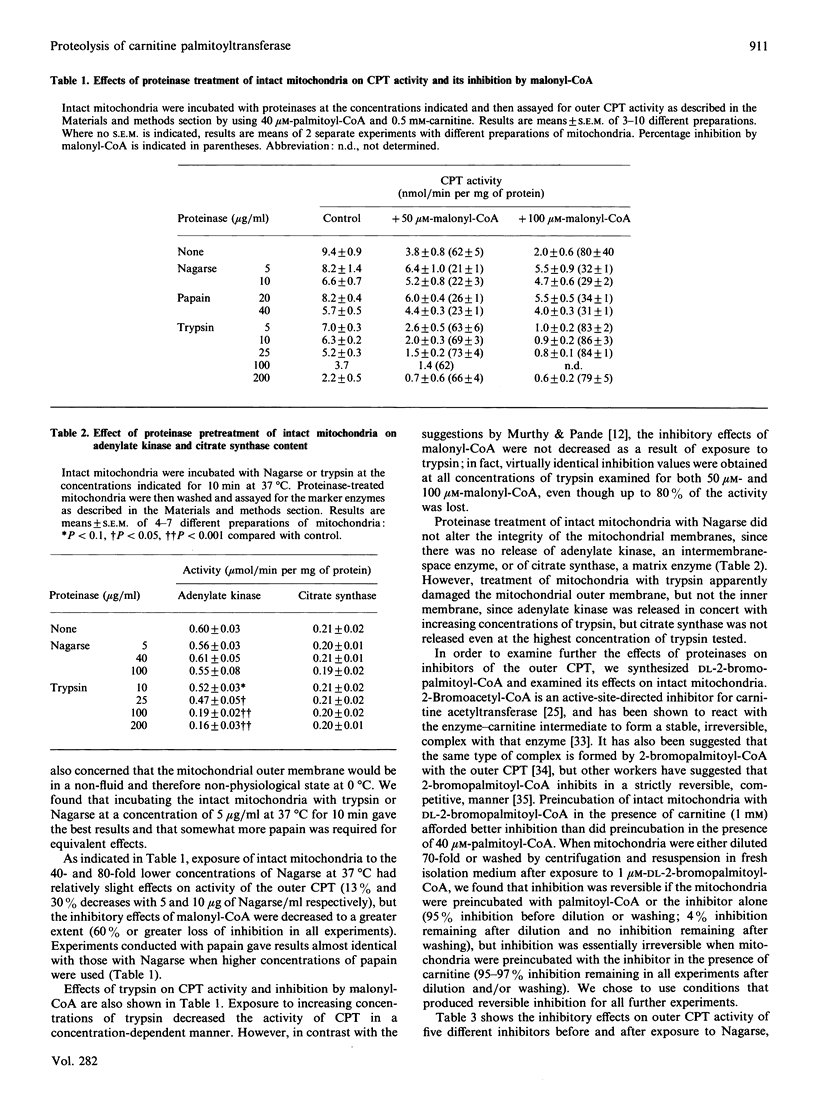
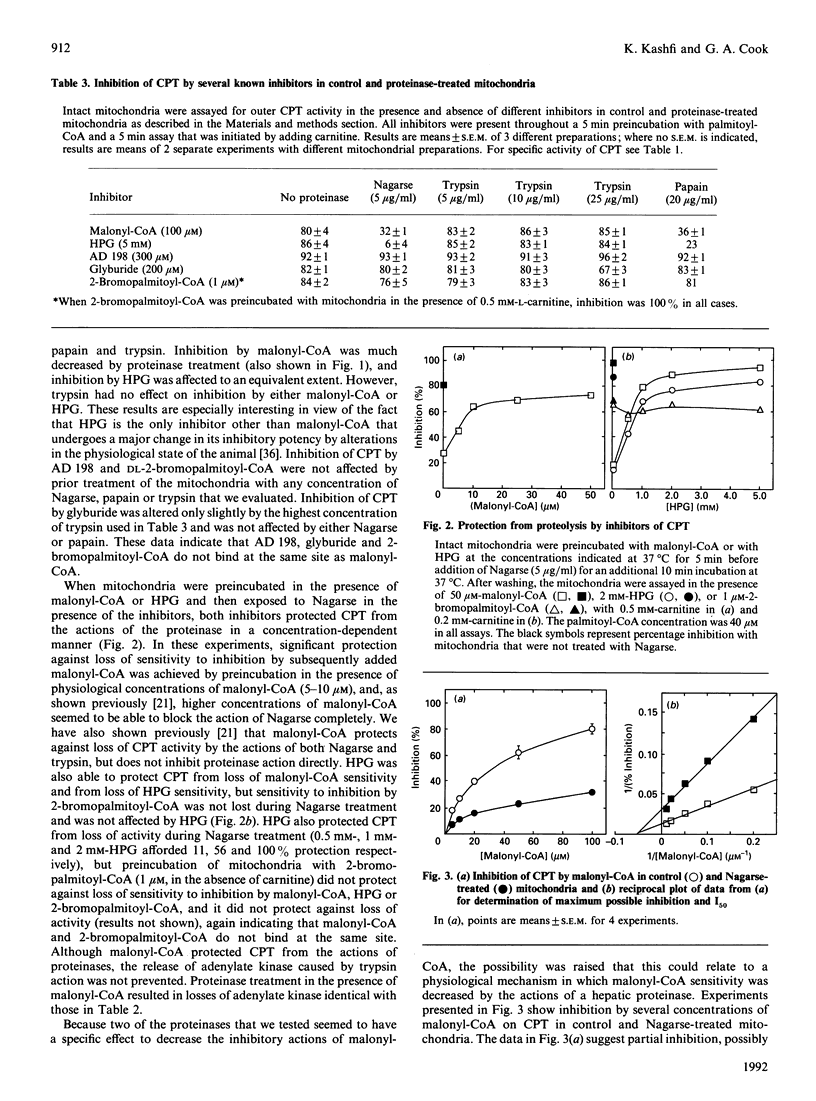
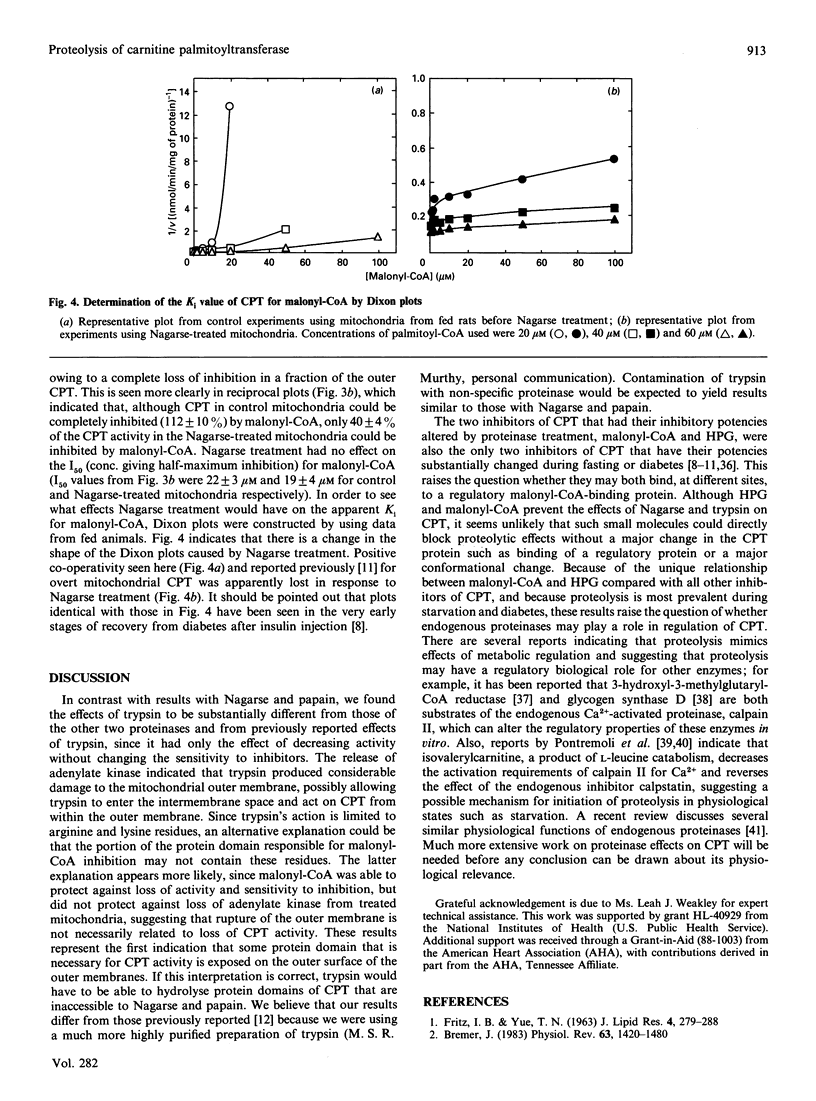
Proteolysis of intact mitochondria by Nagarse (subtilisin BPN') and papain resulted in limited loss of activity of the outer-membrane carnitine palmitoyltransferase, but much greater loss of sensitivity to inhibition by malonyl-CoA. In contrast with a previous report [Murthy & Pande (1987) Proc. Natl. Acad. Sci. U.S.A. 84, 378-382], we found that trypsin had no effect on malonyl-CoA sensitivity. Even when 80% of activity was destroyed by trypsin, there was no difference in the malonyl-CoA sensitivity of the enzyme remaining. Trypsin caused release of the intermembrane-space enzyme adenylate kinase, indicating loss of integrity of the mitochondrial outer membrane, whereas Nagarse and papain caused no release of that enzyme. Citrate synthase was not released by any of the three proteinases, indicating no damage to the mitochondrial inner membrane. When we examined the effects of proteolysis on the inhibition of carnitine palmitoyltransferase by a wide variety of inhibitors having different mechanisms of inhibition, we found differential proteolytic effects that were specific for those inhibitors (malonyl-CoA and hydroxyphenylglyoxylate) that have their inhibitory potencies diminished by changes in physiological state. Both of those inhibitors protected carnitine palmitoyltransferase from the effects of proteolysis, but did not inhibit the proteinases directly. Inhibition by two other inhibitors (DL-2-bromopalmitoyl-CoA and N-benzyladriamycin 14-valerate) was not altered by proteinase treatment, even when most of the enzyme activity had been destroyed. Inhibition by glyburide, which is minimally affected by physiological state, was affected only to a slight extent at the highest concentration of trypsin tested. Proteolysis by Nagarse appeared to produce loss of co-operativity in malonyl-CoA inhibition. The effects of proteolysis are discussed and compared with changes in Ki occurring with changing physiological states.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bieber L. L. Carnitine. Annu Rev Biochem. 1988;57:261–283. doi: 10.1146/annurev.bi.57.070188.001401. [DOI] [PubMed] [Google Scholar]
- Bird M. I., Saggerson E. D. Binding of malonyl-CoA to isolated mitochondria. Evidence for high- and low-affinity sites in liver and heart and relationship to inhibition of carnitine palmitoyltransferase activity. Biochem J. 1984 Sep 15;222(3):639–647. doi: 10.1042/bj2220639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady P. S., Dunker A. K., Brady L. J. Characterization of hepatic carnitine palmitoyltransferase. Use of bromoacyl derivatives and antibodies. Biochem J. 1987 Feb 1;241(3):751–757. doi: 10.1042/bj2410751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer J. Carnitine--metabolism and functions. Physiol Rev. 1983 Oct;63(4):1420–1480. doi: 10.1152/physrev.1983.63.4.1420. [DOI] [PubMed] [Google Scholar]
- Bremer J. The effect of fasting on the activity of liver carnitine palmitoyltransferase and its inhibition by malonyl-CoA. Biochim Biophys Acta. 1981 Sep 24;665(3):628–631. doi: 10.1016/0005-2760(81)90282-4. [DOI] [PubMed] [Google Scholar]
- Chase J. F., Tubbs P. K. Conditions for the self-catalysed inactivation of carnitine acetyltransferase. A novel form of enzyme inhibition. Biochem J. 1969 Jan;111(2):225–235. doi: 10.1042/bj1110225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase J. F., Tubbs P. K. Specific alkylation of a histidine residue in carnitine acetyltransferase by bromoacetyl-L-carnitine. Biochem J. 1970 Feb;116(4):713–720. doi: 10.1042/bj1160713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase J. F., Tubbs P. K. Specific inhibition of mitochondrial fatty acid oxidation by 2-bromopalmitate and its coenzyme A and carnitine esters. Biochem J. 1972 Aug;129(1):55–65. doi: 10.1042/bj1290055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook G. A. Differences in the sensitivity of carnitine palmitoyltransferase to inhibition by malonyl-CoA are due to differences in Ki values. J Biol Chem. 1984 Oct 10;259(19):12030–12033. [PubMed] [Google Scholar]
- Cook G. A., Gamble M. S. Regulation of carnitine palmitoyltransferase by insulin results in decreased activity and decreased apparent Ki values for malonyl-CoA. J Biol Chem. 1987 Feb 15;262(5):2050–2055. [PubMed] [Google Scholar]
- Cook G. A., Otto D. A., Cornell N. W. Differential inhibition of ketogenesis by malonyl-CoA in mitochondria from fed and starved rats. Biochem J. 1980 Dec 15;192(3):955–958. doi: 10.1042/bj1920955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook G. A. The hypoglycemic sulfonylureas glyburide and tolbutamide inhibit fatty acid oxidation by inhibiting carnitine palmitoyltransferase. J Biol Chem. 1987 Apr 15;262(11):4968–4972. [PubMed] [Google Scholar]
- Croall D. E., DeMartino G. N. Calcium-activated neutral protease (calpain) system: structure, function, and regulation. Physiol Rev. 1991 Jul;71(3):813–847. doi: 10.1152/physrev.1991.71.3.813. [DOI] [PubMed] [Google Scholar]
- Declercq P. E., Venincasa M. D., Mills S. E., Foster D. W., McGarry J. D. Interaction of malonyl-CoA and 2-tetradecylglycidyl-CoA with mitochondrial carnitine palmitoyltransferase I. J Biol Chem. 1985 Oct 15;260(23):12516–12522. [PubMed] [Google Scholar]
- FRITZ I. B., YUE K. T. LONG-CHAIN CARNITINE ACYLTRANSFERASE AND THE ROLE OF ACYLCARNITINE DERIVATIVES IN THE CATALYTIC INCREASE OF FATTY ACID OXIDATION INDUCED BY CARNITINE. J Lipid Res. 1963 Jul;4:279–288. [PubMed] [Google Scholar]
- Gamble M. S., Cook G. A. Alteration of the apparent Ki of carnitine palmitoyltransferase for malonyl-CoA by the diabetic state and reversal by insulin. J Biol Chem. 1985 Aug 15;260(17):9516–9519. [PubMed] [Google Scholar]
- Grantham B. D., Zammit V. A. Binding of [14C]malonyl-CoA to rat liver mitochondria after blocking of the active site of carnitine palmitoyltransferase I. Displacement of low-affinity binding by palmitoyl-CoA. Biochem J. 1986 Jan 15;233(2):589–593. doi: 10.1042/bj2330589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham B. D., Zammit V. A. Binding of [14C]malonyl-CoA to rat liver mitochondria after blocking of the active site of carnitine palmitoyltransferase I. Displacement of low-affinity binding by palmitoyl-CoA. Biochem J. 1986 Jan 15;233(2):589–593. doi: 10.1042/bj2330589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga A., Tsuiki S. Activation of a D-form of rabbit muscle glycogen synthase by Ca2+-activated protease. FEBS Lett. 1986 Sep 1;205(1):1–5. doi: 10.1016/0014-5793(86)80853-5. [DOI] [PubMed] [Google Scholar]
- Janski A. M., Cornell N. W. Subcellular distribution of enzymes determined by rapid digitonin fractionation of isolated hepatocytes. Biochem J. 1980 Feb 15;186(2):423–429. doi: 10.1042/bj1860423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashfi K., Cook G. A. Malonyl-CoA inhibits proteolysis of carnitine palmitoyltransferase. Biochem Biophys Res Commun. 1991 Jul 31;178(2):600–605. doi: 10.1016/0006-291x(91)90150-6. [DOI] [PubMed] [Google Scholar]
- Kashfi K., Israel M., Sweatman T. W., Seshadri R., Cook G. A. Inhibition of mitochondrial carnitine palmitoyltransferases by adriamycin and adriamycin analogues. Biochem Pharmacol. 1990 Oct 1;40(7):1441–1448. doi: 10.1016/0006-2952(90)90438-q. [DOI] [PubMed] [Google Scholar]
- Kiorpes T. C., Hoerr D., Ho W., Weaner L. E., Inman M. G., Tutwiler G. F. Identification of 2-tetradecylglycidyl coenzyme A as the active form of methyl 2-tetradecylglycidate (methyl palmoxirate) and its characterization as an irreversible, active site-directed inhibitor of carnitine palmitoyltransferase A in isolated rat liver mitochondria. J Biol Chem. 1984 Aug 10;259(15):9750–9755. [PubMed] [Google Scholar]
- Kolodziej M. P., Zammit V. A. Re-evaluation of the interaction of malonyl-CoA with the rat liver mitochondrial carnitine palmitoyltransferase system by using purified outer membranes. Biochem J. 1990 Apr 1;267(1):85–90. doi: 10.1042/bj2670085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- Mills S. E., Foster D. W., McGarry J. D. Interaction of malonyl-CoA and related compounds with mitochondria from different rat tissues. Relationship between ligand binding and inhibition of carnitine palmitoyltransferase I. Biochem J. 1983 Jul 15;214(1):83–91. doi: 10.1042/bj2140083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy M. S., Pande S. V. Malonyl-CoA binding site and the overt carnitine palmitoyltransferase activity reside on the opposite sides of the outer mitochondrial membrane. Proc Natl Acad Sci U S A. 1987 Jan;84(2):378–382. doi: 10.1073/pnas.84.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy M. S., Pande S. V. Some differences in the properties of carnitine palmitoyltransferase activities of the mitochondrial outer and inner membranes. Biochem J. 1987 Dec 15;248(3):727–733. doi: 10.1042/bj2480727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R. A., Miller S. J., Gibson D. M. Phosphorylation of native 97-kDa 3-hydroxy-3-methylglutaryl-coenzyme A reductase from rat liver. Impact on activity and degradation of the enzyme. J Biol Chem. 1989 Mar 25;264(9):4877–4887. [PubMed] [Google Scholar]
- Pontremoli S., Melloni E., Michetti M., Sparatore B., Salamino F., Siliprandi N., Horecker B. L. Isovalerylcarnitine is a specific activator of calpain of human neutrophils. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1189–1195. doi: 10.1016/s0006-291x(87)80258-9. [DOI] [PubMed] [Google Scholar]
- Pontremoli S., Melloni E., Viotti P. L., Michetti M., Di Lisa F., Siliprandi N. Isovalerylcarnitine is a specific activator of the high calcium requiring calpain forms. Biochem Biophys Res Commun. 1990 Feb 28;167(1):373–380. doi: 10.1016/0006-291x(90)91775-n. [DOI] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A. Effects of fasting and malonyl CoA on the kinetics of carnitine palmitoyltransferase and carnitine octanoyltransferase in intact rat liver mitochondria. FEBS Lett. 1981 Sep 28;132(2):166–168. doi: 10.1016/0014-5793(81)81152-0. [DOI] [PubMed] [Google Scholar]
- Stephens T. W., Harris R. A. Effect of starvation and diabetes on the sensitivity of carnitine palmitoyltransferase I to inhibition by 4-hydroxyphenylglyoxylate. Biochem J. 1987 Apr 15;243(2):405–412. doi: 10.1042/bj2430405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens T. W., Higgins A. J., Cook G. A., Harris R. A. Two mechanisms produce tissue-specific inhibition of fatty acid oxidation by oxfenicine. Biochem J. 1985 Apr 15;227(2):651–660. doi: 10.1042/bj2270651. [DOI] [PMC free article] [PubMed] [Google Scholar]


