Abstract
Chronic neuropathic pain (CNP) remains a significant clinical challenge, with complex neurophysiological underpinnings that are not fully understood. Identifying specific neural oscillatory patterns related to pain perception and interference can enhance our understanding and management of CNP. To analyze resting electroencephalography data from individuals with chronic neuropathic pain to explore the possible neural signatures associated with pain intensity, pain interference, and specific neuropathic pain characteristics. We conducted a secondary analysis from a cross-sectional study using electroencephalography data from a previous study, and Brief Pain Inventory from 36 patients with chronic neuropathic pain. For statistical analysis, we modeled a linear or logistic regression by dependent variable for each model. As independent variables, we used electroencephalography data with such brain oscillations: as delta, theta, alpha, and beta, as well as the oscillations low alpha, high alpha, low beta, and high beta, for the central, frontal, and parietal regions. All models tested for confounding factors such as age and medication. There were no significant models for Pain interference in general activity, walking, work, relationships, sleep, and enjoyment of life. However, the model for pain intensity during the past four weeks showed decreased alpha oscillations, and increased delta and theta oscillations were associated with decreased levels of pain, especially in the central area. In terms of pain interference in mood, the model showed high oscillatory Alpha signals in the frontal and central regions correlated with mood impairment due to pain. Our models confirm recent findings proposing that lower oscillatory frequencies, likely related to subcortical pain sources, may be associated with brain compensatory mechanisms and thus may be associated with decreased pain levels. On the other hand, higher frequencies, including alpha oscillations, may disrupt top-down compensatory mechanisms.
Keywords: pain, EEG, oscillation, chronic neuropathic pain
INTRODUCTION
Chronic pain is a complex condition that is associated with changes in neural processing and can affect the brain’s oscillatory patterns. Chronic neuropathic pain (CNP) is the direct result of a lesion or disease of the nervous system that persists or recurs for more than three months (Bussa et al. 2021; Hange et al. 2022; Treede et al. 2008). According to recent data, CNP is found in about 10% to 30% of adults (Chenaf et al. 2018; van Hecke et al. 2014; M. Zolezzi et al. 2023) and up to 6% of infants (BHATIA et al. 2008).
The diagnosis and treatment of CNP remains a challenge for clinicians today. One of the reasons for this is the lack of objective pain measures for diagnosis, prognosis, and treatment monitoring, which shows that more studies are needed to characterize biomarkers as measurable and objective indicators of CNP. In the meantime, the study of pain may include some techniques to measure sensory thresholds, record electrophysiologic potentials, or assess changes in neuroimaging in response to nociceptive stimuli. These techniques are more relevant for the investigation of provoked pain; spontaneous pain should be investigated using functional neuroimaging or neurophysiological techniques such as magnetoencephalography or electroencephalography (EEG) (Mussigmann, Bardel, and Lefaucheur 2022). A valid biomarker must be based on pattern recognition of brain activity correlated with pain (Levitt and Saab 2019) and help to make the diagnosis more objective and can be associated with pain intensity to improve prognosis by predicting response to an analgesic procedure (Mackey, Greely, and Martucci 2019; van der Miesen, Lindquist, and Wager 2019; Ploner and May 2018; Reckziegel et al. 2019).
A systematic literature review identified 14 resting-state electroencephalographic (rsEEG) studies of patients with neuropathic pain and CNP (Mussigmann et al. 2022). Results found that the presence of pain was associated with increased power in the theta and high beta brain oscillations, while power in high alpha and low beta decreased. However, this meta-analysis showed no consistent trend when analyzing the relationship with pain intensity. Another study involving 38 patients with spinal cord injury has shown that higher alpha power is associated with higher pain intensity measured at frontal electrode sites (Jensen et al. 2013). A systematic review of data from cross-sectional studies, longitudinal studies, and clinical trials found that spectral rsEEG can be considered a simple and objective tool for the study of brain mechanisms involved in chronic pain (Pacheco-Barrios et al. 2023; Pinheiro et al. 2016; Ploner and May 2018; Zebhauser, Hohn, and Ploner 2023).
Despite numerous studies and systematic reviews with significant results showing EEG as a biomarker for pain, we agree with Zis et al. (2022). There is still a gap in classifying EEG as a robust biomarker for pain perception. Although it has potential to be explored, our hypothesis in our current study is: i) there are potential EEG biomarkers for CNP related to areas involved in pain circuits, such as central, frontal, and parietal; ii) EEG markers in CNP can be helpful to understand mechanisms of brain compensatory mechanisms in pain.
Therefore, the present study aims to analyze rsEEG data from individuals diagnosed with CNP and to investigate the neural EEG signatures associated with pain.
METHODS
Subjects
The data used in this article comes from a previous study published by Zolezzi et al. (2023) and analyzed to find EEG brain oscillations as predictors to pain in neuropathic pain. The present analysis is a cross-sectional study.
Sample size
Thirty-six CNP patients calculated two-tailed Z distribution using MATLAB R2020a. The sample size was calculated by Zolezzi et al. (2023) performed in MATLAB R2020a (The Mathworks, Inc., Natick, MA, USA) with the Statistics and Machine Learning Toolbox. Resulting in 32 CNP patients reaching significant differences between CNP and a control group. For a power of 0.9, ß = 0.1 and = 0.05, n = 43. Thus, the objective was to recruit 32 to 43 CNP patients.
Brief Pain Inventory answers
Pain was measured using the Brief Pain Inventory, a self-administered questionnaire with nine items. The questionnaire focuses on the intensity and impact of pain on functioning in the areas of general activity, mood, ability to walk, normal work (including housework), relationships with other people, sleep, and enjoyment of life (Poquet and Lin 2016).
To analyze the associated rsEEG with the severity and interference of pain, we considered the items or questions as primary outcome measures and higher scores meant worse pain. Regarding Brief Pain Inventory Interference items we considered: General activity, Mood, Walking ability, Normal work (including housework), relationships with other people, and Sleep.
Also, for measures directly related to pain, it was considered Pain score during EEG recording, and average Pain intensity in the last 4 weeks. Each question is measured on an 11-point scale – from 0 to 10 – where 0 is “without pain” or “no pain interference”, and 10 is “worst pain” or “complete interference” related to the question.
EEG data
Preprocessing
rsEEG was recorded during ten minutes of spontaneity. In the first five minutes, patients were asked to keep their eyes open and fixed on a white cross in the dark background of a monitor 50 cm away. In the last 5 minutes, the collection was performed with closed eyes until an auditory beep marked the end. EEG data was collected in a 24-channel EASYCAP electrode cap by the 10/20 international system, having M1 and M2 as references (EASYCAP GmbH, Herrsching, Germany). Considering the purpose of the present article, we only considered the eyes closed period for the following analysis.
EEG processing was according to our previous studies (Marques et al. 2022, 2023), the original data were pre-processed to correct any biological or nonbiological artifact that compromised the analyses, using EEGLab (sourced from Swartz Center for Computational Neuroscience, San Diego, California, USA) in MATLAB. Thus, using the Darbeliai EEGLAB plugin (open-source, developed by Dr. Gareth Roberts, available on GitHub) we performed Makoto’s preprocessing pipeline: i) Bandpass of 1 Hz (high pass) and 50 Hz (low pass); ii) downsampling from 1000 Hz to 250 Hz; iii) channels were re-referenced by the average of the electrodes; iv) 60 Hz power line noise correction (frequency in Mexico). Next, using the Clean_rawdata EEGLAB plugin (v2.2, open-source, developed by the Swartz Center for Computational Neuroscience at UC San Diego, hosted on GitHub) we excluded the channels that: i) were flat for more than 3 seconds; present high-frequency noise greater than 2 standard deviations; and iii) present correlation with nearby channels lower than 0.8. Before proceeding to perform the independent component analysis, a visual inspection and rejection of periods with artifacts was performed and it was also validated by a neurophysiologist. The remaining channels were again submitted to the Darbeliai plugin, now for the Infomax ICA7 calculation (algorithm implemented in EEGLab). This analysis is effective in identifying artifacts and is significantly related to other techniques, showing greater efficiency in identifying components. Components related to eye movement, blinking, heart rate, and muscle noise were excluded using the ICLabel toolbox (developed by the Swartz Center for Computational Neuroscience, San Diego, California, USA).
Resting-state spectral power analysis
After all the previously described pre-processing steps had been carried out, the artifact-free data was processed using the pop_spectopo EEGLab function with Fast Fourier Transformation with 5-second windows with 50% overlap. Relative power (power in a specific frequency range/total power from 1 to 30 Hz) was calculated for the following oscillations: delta (1–3.9 Hz), theta (4–7.9 Hz), alpha (8–12.9 Hz), beta (13–29.9 Hz), and the sub-oscillations: low alpha (8–9.9 Hz), high alpha (10–12.9 Hz), low beta (13–19.9 Hz) and high beta (20–30 Hz) by brain region, being excluded in our present study the occipital and temporal brain region because of the lack of plausible relationship with pain circuits.
Clinical assessment and demographic data
In our actual study, we consider only the Brief Pain Inventory and the demographic data to analyze those: age, gender, etiology and time of neuropathic pain, and medications (Table 1).
Table 1:
Characteristics of the participants.
| Variables | Data |
|---|---|
|
| |
| Population (n) | 36 |
| Age (yr) | 44.00±13.98 |
| Sex | |
| Male | 8 (22) |
| Female | 28 (78) |
| Pain category | |
| Mild | 17 (47) |
| Severe | 19 (53) |
| Pain category in last 4 weeks | |
| Mild | 12 (33) |
| Severe | 24 (67) |
| Chronicity | |
| 3–6 mon | 1 (3) |
| 6–12 mon | 2 (6) |
| 12– 24 mon | 6 (17) |
| >24 mon | 27 (75) |
| Etiology | |
| Central nervous system disorder | 3 (8) |
| Trigeminal neuralgia | 3 (8) |
| Diabetes | 6 (17) |
| Peripheral neuropathy | 10 (28) |
| Spinal cord/nerve root injury | 11 (30) |
| Other | 3 (8) |
| Medication | |
| No treatment | 14 (39) |
| Mixed | 7 (19) |
| Opioid | 4 (11) |
| Anticonvulsivant | 7 (19) |
| Antidepressant | 2 (6) |
| Others | 2 (6) |
Note: All data are expressed as number (percentage), except age as mean ± SD.
Statistical analysis
All the statistical analyses were made using Stata software (version 17.0). To investigate the neural signatures associated with pain intensity, pain interference, and specific neuropathic pain characteristics we performed Linear and Logistic regression according to the characteristic of dependent variable - linear regression for continuous variables and logistic regression for categorical variables.
We used the following dependent variables: (i) the pain intensity during the EEG recording; (ii) the average pain intensity during the past four weeks; (iii) Pain interference in general activity; (iv) Pain interference in mood; (v) Pain interference in walking ability; (vi) Pain interference in work; (vii) Pain interference in relationships; (viii) Pain interference in sleep; and (ix) Pain interference in enjoyment life.
Independent variables were brain oscillations, considering here the central, frontal, and parietal regions and the following oscillations: delta, theta, alpha, beta, and the sub oscillations low alpha, high alpha, low beta, high beta.
Normality assessment
To decide which type of regression would be run, all the dependent variables were assessed by normality through histogram (Additional Table 1).
There was one variable identified as normal, which was “Pain interference on mood” and for that, we ran linear regression. All the other variables were seen to be not normally distributed, and we performed logistic regression after categorizing those variables and were categorized by the median (categorized as “0” if the value is ≤ median; and “1” if > median).
Univariate analysis
For the univariate analysis, we considered less than equal P-value < 0.2 in the model to be considered in the next step and to find confounders for analysis. The independent variables were chosen based on a criterion and biological plausibility (Bursac et al. 2008) being one of the reasons to take off the analysis of brain oscillatory from the occipital and temporal region. We provide in the Supplementary material all the univariate analysis results.
Multivariate analysis
The multivariate analysis were performed controlled by age and medication (Table 1) as confounders, in consonance with previous EEG studies that mentioned the potential of these variables to interfere with patterns of brain oscillations (Anderson and Perone 2018; Galderisi and Sannita 2006).
Ethical aspects
According to Zolezzi et al. (2023) before the start of the experiment, all patients provided written informed consent according to the World Association Declaration of Helsinki. This study was approved by the ethics committee of the Clinical Investigation Ethics Committee of Tecnologico de Monterrey (No. P000369-DN-RespElectro-CI-CR005). The data used in our analysis, as mentioned previously, were provided by the authors and are available in a publicly accessible database.
RESULTS
A summary of the main characteristics of the patients can be found in Table 1. Our population consisted of 36 patients with CNP, 28 females, and the mean age was 44 ± 13.98 years old. Regarding pain category, the group is balanced being 17 with moderate pain and 19 with severity pain score. However, regarding pain reported in the last 4 weeks, 12 patients are mild and 24 in more severe pain. Regarding medication, a potential confounder of the EEG, 14 patients did not use any medication and about 75% of the sample has had CNP for more than 24 months.
The relative power of brain oscillations is described in Table 2.
Table 2:
A summary of relative power (%) electroencephalography, sorted by region of the scalp
| Central | Frontal | Parietal | |
|---|---|---|---|
|
| |||
| Delta | 0.221±0.792 | 0.178±0.083 | 0.130±0.072 |
| Theta | 0.200±0.076 | 0.214±0.076 | 0.187±0.079 |
| Alpha | |||
| Alpha | 0.401±0.141 | 0.405±0.154 | 0.161±0.163 |
| Low alpha | 0.199±0.107 | 0.204±0.118 | 0.233±0.129 |
| High alpha | 0.191±0.083 | 0.189±0.095 | 0.234±0.109 |
| Beta | |||
| High beta | 0.074±0.038 | 0.074±0.038 | 0.062±0.032 |
| Low beta | 0.134±0.042 | 0.110±0.045 | 0.120±0.046 |
The univariate analysis for each dependent variable was performed. For the pain score, only brain oscillations in the central region reached our cut-off P-value (low beta, central beta, and central high beta). For the dependent variable, the pain score in the last 4 weeks in the central region was low alpha, alpha, high alpha, low beta, beta, high beta, and delta. In the frontal region, low alpha, alpha, low beta, beta, high beta, and delta. Pain score in the last 4 weeks in the parietal region had an association with low alpha, alpha, low beta, beta, high beta, and delta (Additional Table 1).
Interference in the general activity had an association in the central region with low alpha, alpha, high alpha, low beta, beta, high beta, and delta; for frontal, association with low alpha, alpha, low beta, beta, high beta, delta. In the parietal low alpha, alpha, low beta, beta, high beta and delta.
Regarding the interference in the mood, our unique variables that used ran logistic regression, the variables that reached our cut-off in this step showing association in the central region were low alpha, alpha, high alpha, low beta, beta, high beta and delta. In the frontal, the association was shown with alpha, low beta, beta, and high beta. The parietal had an association with alpha, low beta, beta, and high beta.
Concerning the sleep interference, univariate analysis showed association only with low beta in the parietal region, and for interference in the enjoyment of life only alpha in the central region had significance.
Multivariate analysis
Multivariate analyses were performed following the forward stepwise, biological plausibility, and results from previous EEG pain-related publications (Pacheco-Barrios et al. 2023; Ploner and May 2018; Zebhauser et al. 2023). The descriptive variables such as age and medication were tested in the final model to see if they are confounders or effect modifiers.
Regarding the Pain intensity during the EEG recording, Pain interference in general activity, Pain interference in walking ability, Pain interference in work, Pain interference in relationships, Pain interference in sleep, and Pain interference in enjoyment of life we did not find any significant model.
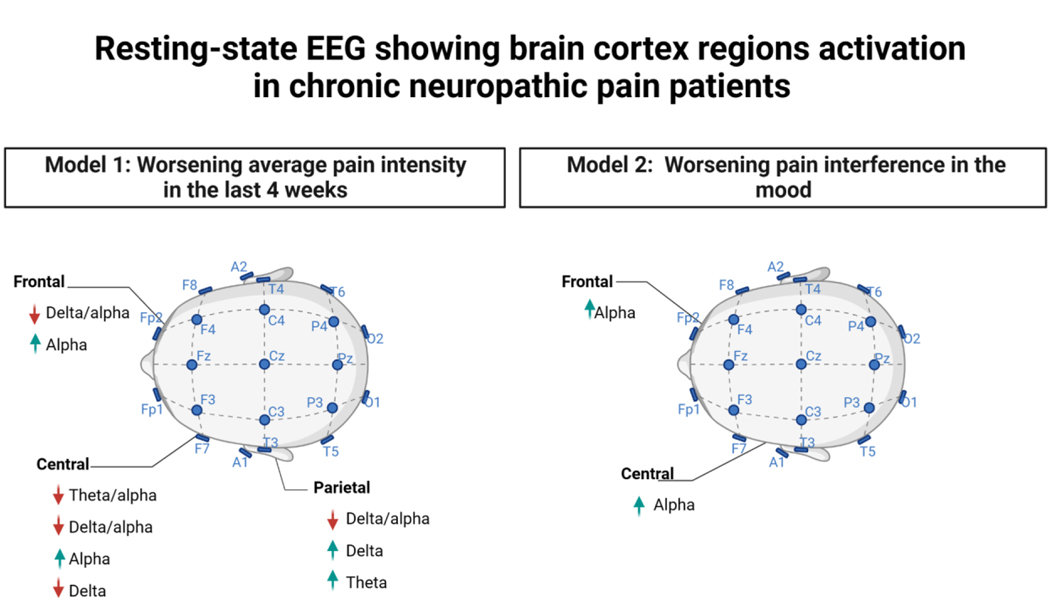
Considering the average pain intensity during the past four weeks and Pain interference on mood, we find a significant multivariate model, as is shown in Tables 3 (Model 1) and 4 (Model 2), respectively. Regarding the level of pain, in the central region, alpha oscillations were linked to higher levels of pain and delta to lower levels. Higher alpha in the frontal region was similar to the central region (more alpha, more pain), and Delta and Theta in the frontal region were similar (less delta and theta, less pain) (Figure 1). The relationship was flipped in the parietal region: more delta was linked to higher pain intensity and more alpha and theta to decreased pain intensity. Neither pain nor medication was a confounding variable in these models.
Table 3:
Model 1 - Average Pain intensity in the last 4 weeks
| Odds ratio | 95% confidence interval | P-value | ROC | |
|---|---|---|---|---|
|
| ||||
| Central | ||||
| Alpha | 87990 | 24.064 – 0.000 | 0.007 | 0.826 |
| Delta | 0.000 | 0.721 – 2.882 | 0.004 | 0.870 |
| Theta | 0.001 | 0.000 – 73.46 | 0.243 | 0.684 |
| Frontal | ||||
| Alpha | 9148.98 | 2.680 – 312217 | 0.022 | 0.767 |
| Delta | 0.028 | 0.000 – 480 | 0.483 | 0.6502 |
| Theta | 0.007 | 0.000 – 87.2 | 0.305 | 0.6780 |
| Parietal | ||||
| Alpha | 0.000 | 0.000 – 0.020 | 0.795 | 0.795 |
| Delta | 1125.79 | 4.197 – 301923 | 0.014 | 0.792 |
| Theta | 0.000 | 0.000 – 0.004 | 0.010 | 0.805 |
Note: Model 1 was tested for age and medication. ROC: receiver operating characteristic curve.
Figure 1:
Resting-state EEG showing multiple brain cortex regions activation in chronic neuropathic pain patients.
It is worth noting did not find a model consisting of Beta oscillations, even an association with the pain interference on the mood, even though Beta oscillations have shown association in the univariate step.
Interference in the mood (Table 4), we could model only the central and frontal regions, showing the delta and theta brain oscillations with an inversely proportional relationship in the interference of mood. In the frontal region, only theta oscillations kept the inverted relationship.
Table 4:
Model 2 - Pain interference in the mood
| Coefficient | 95% Confidence interval | P-value | R-adjusted | AIC | |
|---|---|---|---|---|---|
|
| |||||
| Central | |||||
| Alpha | 7.58 | 1.17 – 14.00 | 0.02 | 0.10 | 1.75 |
| Delta | −12.23 | −24.78 – 0.31 | 0.05 | 0.06 | 177.22 |
| Theta | −5.76 | −20.32 – 8.80 | 0.42 | −0.03 | 1.80 |
| Frontal | |||||
| Alpha | 6.40 | 0.33 – 12.47 | 0.03 | 0.08 | 1.76 |
| Delta | 6.53 | −21.73 – 1.97 | 0.09 | 0.03 | 178.30 |
| Theta | −7.71 | −21.30 – 5.87 | 0.25 | − 0.01 | 179.93 |
Note: Model 2 was tested for age and medication. AIC: Akaike Information Criterion (AIC).
DISCUSSION
In our study, we investigate possible associations between rsEEG and pain intensity, pain interference, and neuropathic pain characteristics in CNP patients. Given our chosen targets, we excluded the occipital and temporal brain oscillatory regions of the scalp, as these areas are not associated with the pain pathway (De Ridder, Adhia, and Vanneste 2021). The first important observation is that average pain in 4 weeks was the variable that provided the best model. All the interference pain variables, except for the interference on mood, did not result in significant models. The pain interference on the mood model provided a model but not as strong as the pain average 4-week model. Also, another important observation is that the most consistent area was the central area for the EEG oscillations associated with pain outcomes.
Alpha oscillations
In our results, Alpha oscillations in the Central region have been associated with higher pain referred in the last 4 weeks in patients with CNP. In addition, High Alpha oscillation has already been associated with neuropathic pain (Kisler et al. 2020). It is worth noting the role of Alpha in healthy conditions, as a dominant rhythm in the awake brain and is known to play an important role in pain states (Fauchon et al. 2022) which was shown in our current result and in agreement with other studies which suggest that the brain alpha rhythm in pain perception seems to be compensatory, in case of chronic pain (Schmiedt-Fehr, Mathes, and Basar-Eroglu 2009).
The increased occurrence of Alpha oscillations in the Frontal area is associated with more pain stimuli in the last 4 weeks and may represent responses from the cognitive processing of pain stimuli (Lorenz, Minoshima, and Casey 2003; Zhou et al. 2020). As for the Alpha oscillations in the parietal region, they are probably related to the intersensory reorientation in pain (Misselhorn, Friese, and Engel 2019), which has been demonstrated in chronic pain.
Even though other studies reported that an increase in Alpha power in the frontal region could lead to improved pain modulation and cognitive control over pain perception (Rustamov et al. 2022) and the emotional impact of chronic pain. It is possible that an increase in alpha power in this region could lead to improved pain modulation and cognitive control over pain perception, considering that alpha power can act as a top-down inhibitory oscillation to pain (Ahmed, Jones, and Sivan 2020), so our current results should be interpreted with caution. However, there does not appear to be a unanimous consensus on alpha in this region, as another study of patients with chronic pain associated with multiple sclerosis showed abnormalities in spectral power within regions of the dynamic pain connectome (Kim et al. 2019).
As far as the parietal region is described in our results, Alpha and Theta oscillations were associated with a lower perception of pain in the last 4 weeks and Delta was more associated with high pain intensity in the last 4 weeks.
Theta oscillations
Changes in Theta power and connectivity may be associated with frontal brain regions in the pathophysiology of chronic pain involving attentional processes, emotional regulation, and memory (Ta Dinh et al. 2019). This is supported by studies in which the pain stimulus is processed, and theta is reported to be specifically associated with the preparation and processing phases surrounding the onset of the stimulus (Taesler and Rose 2016). In addition, recent publications from our research group have shown that frontal region Theta represents a compensatory mechanism for the polymorphisms of OPRM1 in patients with knee osteoarthritis (Gonçalves et al. 2023).
Specifically, pain intensity is associated with increased Theta activity in the electrodes over the parietal regions (Camfferman et al. 2017), which is consistent with our results showing that more Theta oscillations are associated with greater change in pain intensity. Individuals with chronic pain may show changes in theta power in frontal regions (Pinheiro et al. 2016; Rustamov et al. 2022) and it may have a connection with compensatory and maladaptive brain rhythms (Simis et al. 2022).
Delta oscillations
Delta oscillation, in the healthy brain, is most evident during slow-wave sleep, and there is a lot of evidence that suggests the involvement of delta oscillation in motivations like an increase during hunger, panic attack, and sustained pain (Knyazev 2012). Besides that, delta power in the resting-state stage has a relationship with the brain’s default mode network which may be altered in patients with pain, especially during chronic processes (Meerwijk, Ford, and Weiss 2015).
In contrast with our findings in the central region, some studies have reported increased delta power at rest in individuals with chronic pain conditions, suggesting that it might be related to the ongoing perception of pain (Pinheiro et al. 2016) which also has plausibility once delta is related to cognitive processes (Harmony 2013).
Also, the relationship between delta-pain seems to be related to active-avoidant control to reduce focused attention on the painful stimulation (De Pascalis et al. 2019). These brain oscillations need to be more present in chronic pain to avoid spikes and unpleasant sensations, which is a possible compensation (De Pascalis et al. 2019).
In other previous studies in patients with chronic pain due to knee osteoarthritis, delta oscillation was associated with higher intracortical inhibition, which is also a signal of compensation (Simis et al. 2022). Also, a study in brain-injured subjects showed that low-frequency brain oscillations are associated with compensatory top-down brain mechanisms and better outcomes (Liu et al. 2023).
Although frontal functions are affected by aging and therefore may influence pain tolerance (Zhou et al. 2020), age was not a confounder in these models either. On the other hand, delta frontal power increases in the acute pain phase (it may be involved in the cognitive and emotional impact of chronic pain). For example, it has already been established that an increase in delta in the frontal region is one of the most reported EEG changes while a painful stimulus is being promoted (Zis et al. 2022), thus suggesting that an increase in delta oscillations may be a compensatory mechanism.
Alpha and beta oscillations
Although our results do not find beta oscillations as a predictor in the final models, it may be timely to cite other studies that associate the increase of frequency maybe because of the relationship between sensory processing of pain (Mussigmann et al. 2022). Besides that, beta oscillations shown associated with pain intensity in patients with knee osteoarthritis in the frontal region (Simis et al. 2022) and shown in patients with fibromyalgia high connectivity and activity associated with beta-3 frequency band involved in process sensory, affective, and attention (Alves et al. 2023). Especially low beta oscillations were associated with polymorphism mu-opioid receptors (Gonçalves et al. 2023).
In general, contradictorily, in another study with CNP was observed pain decrease in alpha and increase in theta, delta, and beta oscillations power, and after electrical spinal cord stimulation normalization of alpha and normalization/significant amplitude decrease in the total spectral range (Sufianov et al. 2014). However, the theta/alpha ratio in the central region seems more correlated with less pain severity in the past 4 weeks.
It has previously been reported that relief of chronic pain was associated with a significant increase in delta, theta, and alpha power in frontal regions (Rustamov et al. 2022). However, this does not seem to apply to all types of pain. In a previous study by our research group, theta oscillations had a negative correlation with pain intensity in patients with knee osteoarthritis in the same frontal region (Simis et al. 2022).
Interference in the mood
Chronic pain is known to have a significant effect on mood, often leading to symptoms of depression and anxiety once the connection between depression and pain seems to be bidirectional (Bair et al. 2003; Loggia, Mogil, and Bushnell 2008; Rayner et al. 2016). The central region, which includes regions such as the primary motor cortex and the somatosensory cortex, is involved in both sensory and motor functions.
Therefore, alpha oscillations are often associated with a state of relaxation. Regarding theta activity may be associated with changes in mood and attentional focus related to pain. EEG oscillations in the central area may reflect the interaction between the sensory aspects of pain processing and that contribute to mood fluctuations. Interventions that consider brain oscillations, such as neurofeedback, can potentially improve mood and pain outcomes, even though the studies dedicated to showing efficacy have a huge variety of approaches and protocols (Patel et al. 2020).
Regarding the clinical impact of our finding, despite our study not being confirmatory, it supports in a way the use of real-time feedback on their brain activity to modulate their neural patterns, which could affect both pain perception and mood. It is worth noting that individual differences may have an important role in how EEG oscillations are related to pain and mood and more studies can address this issue on account people may show different patterns of brain activity in response to pain.
Concerning our study limitations, we consider mainly the sample size and the lack of balance between women and men, which require the use of more sophisticated statistics to analyze the influence of gender in our results.
In conclusion, our results are generally consistent with previous pain EEG studies, despite some differences. EEG alpha and theta oscillations are potential biomarkers assessing average pain intensity in the last 4 weeks and interference of the pain in the mood, in CNP patients.
Supplementary Material
Funding
FF received support from NIH 2020 R01 AT, Project #1R01AT009491-01A1.
Footnotes
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- Ahmed Hasaan, Jones Anthony, and Sivan Manoj. 2020. “The Brain Alpha Rhythm in the Perception and Modulation of Pain.” Advances in Clinical Neuroscience & Rehabilitation 19(4):31–34. doi: 10.47795/GBPD9851. [DOI] [Google Scholar]
- Alves Rael Lopes, Zortea Maxciel, Serrano Paul Vicuña, Tomedi Rafaela Brugnera, de Almeida Rodrigo Pereira, Torres Iraci L. S., Fregni Felipe, and Caumo Wolnei. 2023. “High-Beta Oscillations at EEG Resting State and Hyperconnectivity of Pain Circuitry in Fibromyalgia: An Exploratory Cross-Sectional Study.” Frontiers in Neuroscience 17:1233979. doi: 10.3389/fnins.2023.1233979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson Alana J., and Perone Sammy. 2018. “Developmental Change in the Resting State Electroencephalogram: Insights into Cognition and the Brain.” Brain and Cognition 126:40–52. doi: 10.1016/j.bandc.2018.08.001. [DOI] [PubMed] [Google Scholar]
- Bair Matthew J., Robinson Rebecca L., Katon Wayne, and Kroenke Kurt. 2003. “Depression and Pain Comorbidity.” Archives of Internal Medicine 163(20):2433. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- BHATIA ANUJ, BRENNAN LIAM, ABRAHAMS MARK, and GILDER FAY. 2008. “Chronic Pain in Children in the UK: A Survey of Pain Clinicians and General Practitioners.” Pediatric Anesthesia 18(10):957–66. doi: 10.1111/j.1460-9592.2008.02710.x. [DOI] [PubMed] [Google Scholar]
- Bursac Zoran, Gauss C. Heath, Williams David Keith, and Hosmer David W. 2008. “Purposeful Selection of Variables in Logistic Regression.” Source Code for Biology and Medicine 3(1):17. doi: 10.1186/1751-0473-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussa M, Mascaro A, Sbacchi E, Dourandish M, and Rinaldi S. 2021. “Understanding Peripheral Neuropathic Pain in Primary Care: Diagnosis and Management.” European Review for Medical and Pharmacological Sciences 25(4):1990–96. doi: 10.26355/eurrev_202102_25100. [DOI] [PubMed] [Google Scholar]
- Camfferman Danny, Moseley G. Lorimer, Gertz Kevin, Pettet Mark W., and Jensen Mark P. 2017. “Waking EEG Cortical Markers of Chronic Pain and Sleepiness.” Pain Medicine (Malden, Mass.) 18(10):1921–31. doi: 10.1093/pm/pnw294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenaf Chouki, Delorme Jessica, Delage Noémie, Ardid Denis, Eschalier Alain, and Authier Nicolas. 2018. “Prevalence of Chronic Pain with or without Neuropathic Characteristics in France Using the Capture–Recapture Method: A Population-Based Study.” Pain 159(11):2394–2402. doi: 10.1097/j.pain.0000000000001347. [DOI] [PubMed] [Google Scholar]
- Fauchon Camille, Kim Junseok A., El-Sayed Rima, Osborne Natalie R., Rogachov Anton, Cheng Joshua C., Hemington Kasey S., Bosma Rachael L., Dunkley Benjamin T., Oh Jiwon, Bhatia Anuj, Inman Robert D., and Davis Karen D. 2022. “Exploring Sex Differences in Alpha Brain Activity as a Potential Neuromarker Associated with Neuropathic Pain.” Pain 163(7):1291–1302. doi: 10.1097/j.pain.0000000000002491. [DOI] [PubMed] [Google Scholar]
- Galderisi Silvana, and Sannita Walter G. 2006. “Pharmaco-EEG: A History of Progress and a Missed Opportunity.” Clinical EEG and Neuroscience 37(2):61–65. doi: 10.1177/155005940603700204. [DOI] [PubMed] [Google Scholar]
- Gonçalves, de Toledo Fernanda, Marques Lucas Murrins, Pessotto Anne Victório, Barbosa Sara Pinto, Imamura Marta, Simis Marcel, Fregni Felipe, and Battistella Linamara. 2023. “OPRM1 and BDNF Polymorphisms Associated with a Compensatory Neurophysiologic Signature in Knee Osteoarthritis Patients.” Neurophysiologie Clinique 53(6):102917. doi: 10.1016/j.neucli.2023.102917. [DOI] [PubMed] [Google Scholar]
- Hange Namrata, Poudel Sujan, Ozair Saleha, Paul Trissa, Nambakkam Meghna, Shrestha Rakchhya, Greye Farrah, Shah Sangam, Adhikari Yagya Raj, Thapa Sangharsha, and Patel Pooja. 2022. “Managing Chronic Neuropathic Pain: Recent Advances and New Challenges.” Neurology Research International 2022:1–14. doi: 10.1155/2022/8336561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmony Thalía. 2013. “The Functional Significance of Delta Oscillations in Cognitive Processing.” Frontiers in Integrative Neuroscience 7. doi: 10.3389/fnint.2013.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hecke O, Austin Sophie K., Khan Rafi A., Smith BH, and Torrance N. 2014. “Neuropathic Pain in the General Population: A Systematic Review of Epidemiological Studies.” Pain 155(4):654–62. doi: 10.1016/j.pain.2013.11.013. [DOI] [PubMed] [Google Scholar]
- Jensen MP, Sherlin LH, Gertz KJ, Braden AL, Kupper AE, Gianas A, Howe JD, and Hakimian S. 2013. “Brain EEG Activity Correlates of Chronic Pain in Persons with Spinal Cord Injury: Clinical Implications.” Spinal Cord 51(1):55–58. doi: 10.1038/sc.2012.84. [DOI] [PubMed] [Google Scholar]
- Kim Junseok A., Bosma Rachael L., Hemington Kasey S., Rogachov Anton, Osborne Natalie R., Cheng Joshua C., Oh Jiwon, Crawley Adrian P., Dunkley Ben T., and Davis Karen D. 2019. “Neuropathic Pain and Pain Interference Are Linked to Alpha-Band Slowing and Reduced Beta-Band Magnetoencephalography Activity within the Dynamic Pain Connectome in Patients with Multiple Sclerosis.” Pain 160(1):187–97. doi: 10.1097/j.pain.0000000000001391. [DOI] [PubMed] [Google Scholar]
- Kisler Lee B., Kim Junseok A., Hemington Kasey S., Rogachov Anton, Cheng Joshua C., Bosma Rachael L., Osborne Natalie R., Dunkley Benjamin T., Inman Robert D., and Davis Karen D. 2020. “Abnormal Alpha Band Power in the Dynamic Pain Connectome Is a Marker of Chronic Pain with a Neuropathic Component.” NeuroImage: Clinical 26:102241. doi: 10.1016/j.nicl.2020.102241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knyazev Gennady G. 2012. “EEG Delta Oscillations as a Correlate of Basic Homeostatic and Motivational Processes.” Neuroscience & Biobehavioral Reviews 36(1):677–95. doi: 10.1016/j.neubiorev.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Levitt Joshua, and Saab Carl Y. 2019. “What Does a Pain ‘Biomarker’ Mean and Can a Machine Be Taught to Measure Pain?” Neuroscience Letters 702:40–43. doi: 10.1016/j.neulet.2018.11.038. [DOI] [PubMed] [Google Scholar]
- Liu Sinan, Shi Chaoqun, Meng Huanhuan, Meng Yu, Gong Xin, Chen Xiping, and Tao Luyang. 2023. “Cognitive Control Subprocess Deficits and Compensatory Modulation Mechanisms in Patients with Frontal Lobe Injury Revealed by EEG Markers: A Basic Study to Guide Brain Stimulation.” General Psychiatry 36(4):e101144. doi: 10.1136/gpsych-2023-101144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loggia Marco L., Mogil Jeffrey S., and Bushnell M. Catherine. 2008. “Experimentally Induced Mood Changes Preferentially Affect Pain Unpleasantness.” The Journal of Pain 9(9):784–91. doi: 10.1016/j.jpain.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Lorenz J, Minoshima S, and Casey KL 2003. “Keeping Pain out of Mind: The Role of the Dorsolateral Prefrontal Cortex in Pain Modulation.” Brain 126(5):1079–91. doi: 10.1093/brain/awg102. [DOI] [PubMed] [Google Scholar]
- Zolezzi M, Daniela, Naal-Ruiz Norberto E., Alonso-Valerdi Luz María, and Ibarra-Zarate David I. 2023. “Chronic Neuropathic Pain: EEG Data in Eyes Open and Eyes Closed with PainDETECT and Brief Pain Inventory Reports.” Data in Brief 48:109060. doi: 10.1016/j.dib.2023.109060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey Sean, Greely Henry T., and Martucci Katherine T. 2019. “Neuroimaging-Based Pain Biomarkers: Definitions, Clinical and Research Applications, and Evaluation Frameworks to Achieve Personalized Pain Medicine.” PAIN Reports 4(4):e762. doi: 10.1097/PR9.0000000000000762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques Lucas M., Barbosa Sara P., Pacheco-Barrios Kevin, Goncalves Fernanda T., Imamura Marta, Battistella Linamara R., Simis Marcel, and Fregni Felipe. 2022. “Motor Event-Related Synchronization as an Inhibitory Biomarker of Pain Severity, Sensitivity, and Chronicity in Patients with Knee Osteoarthritis.” Neurophysiologie Clinique 52(6):413–26. doi: 10.1016/j.neucli.2022.09.006. [DOI] [PubMed] [Google Scholar]
- Marques Lucas M., Castellani Ana, Barbosa Sara P., Imamura Marta, Battistella Linamara R., Simis Marcel, and Fregni Felipe. 2023. “Neuroplasticity Changes in Knee Osteoarthritis (KOA) Indexed by Event-Related Desynchronization/Synchronization during a Motor Inhibition Task.” Somatosensory & Motor Research 1–10. doi: 10.1080/08990220.2023.2188926. [DOI] [PubMed] [Google Scholar]
- Meerwijk Esther L., Ford Judith M., and Weiss Sandra J. 2015. “Resting-State EEG Delta Power Is Associated with Psychological Pain in Adults with a History of Depression.” Biological Psychology 105:106–14. doi: 10.1016/j.biopsycho.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Miesen Maite M., Lindquist Martin A., and Wager Tor D. 2019. “Neuroimaging-Based Biomarkers for Pain: State of the Field and Current Directions.” PAIN Reports 4(4):e751. doi: 10.1097/PR9.0000000000000751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misselhorn Jonas, Friese Uwe, and Engel Andreas K. 2019. “Frontal and Parietal Alpha Oscillations Reflect Attentional Modulation of Cross-Modal Matching.” Scientific Reports 9(1):5030. doi: 10.1038/s41598-019-41636-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussigmann Thibaut, Bardel Benjamin, and Lefaucheur Jean-Pascal. 2022. “Resting-State Electroencephalography (EEG) Biomarkers of Chronic Neuropathic Pain. A Systematic Review.” NeuroImage 258:119351. doi: 10.1016/j.neuroimage.2022.119351. [DOI] [PubMed] [Google Scholar]
- Pacheco-Barrios Kevin, Marques Lucas M., Dodurgali Mustafa Reha, Martinez-Magallanes Daniela, Barbosa Sara P., Daibes Marianna, Márquez Jorge Ortega, de Melo Paulo S., Simis Marcel, Caumo Wolnei, and Fregni Felipe. 2023. “Seeking Brain Homeostatic Compensatory Mechanisms for Pain Control.” Principles and Practice of Clinical Research Journal 9(2). doi: 10.21801/ppcrj.2023.92.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascalis De, Vilfredo Paolo Scacchia, Papi Beatrice, and Corr Philip J. 2019. “Changes of EEG Band Oscillations to Tonic Cold Pain and the Behavioral Inhibition and Fight-Flight-Freeze Systems.” Personality Neuroscience 2:e12. doi: 10.1017/pen.2019.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel Kajal, Sutherland Heather, Henshaw James, Taylor Jason R., Brown Christopher A., Casson Alexander J., Trujillo-Barreton Nelson J., Jones Anthony K. P., and Sivan Manoj. 2020. “Effects of Neurofeedback in the Management of Chronic Pain: A Systematic Review and Meta-analysis of Clinical Trials.” European Journal of Pain 24(8):1440–57. doi: 10.1002/ejp.1612. [DOI] [PubMed] [Google Scholar]
- Pinheiro, dos Santos Eulália Silva, de Queirós Fernanda Costa, Montoya Pedro, Santos Cleber Luz, do Nascimento Marion Alves, Ito Clara Hikari, Silva Manuela, Santos David Barros Nunes, Benevides Silvia, Miranda José Garcia Vivas, Sá Katia Nunes, and Baptista Abrahão Fontes. 2016. “Electroencephalographic Patterns in Chronic Pain: A Systematic Review of the Literature.” PLOS ONE 11(2):e0149085. doi: 10.1371/journal.pone.0149085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploner Markus, and May Elisabeth S. 2018. “Electroencephalography and Magnetoencephalography in Pain Research—Current State and Future Perspectives.” Pain 159(2):206–11. doi: 10.1097/j.pain.0000000000001087. [DOI] [PubMed] [Google Scholar]
- Poquet Nolwenn, and Lin Christine. 2016. “The Brief Pain Inventory (BPI).” Journal of Physiotherapy 62(1):52. doi: 10.1016/j.jphys.2015.07.001. [DOI] [PubMed] [Google Scholar]
- Rayner Lauren, Hotopf Matthew, Petkova Hristina, Matcham Faith, Simpson Anna, and McCracken Lance M. 2016. “Depression in Patients with Chronic Pain Attending a Specialised Pain Treatment Centre: Prevalence and Impact on Health Care Costs.” Pain 157(7):1472–79. doi: 10.1097/j.pain.0000000000000542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reckziegel Diane, Vachon-Presseau Etienne, Petre Bogdan, Schnitzer Thomas J., Baliki Marwan N., and Vania Apkarian A. 2019. “Deconstructing Biomarkers for Chronic Pain: Context- and Hypothesis-Dependent Biomarker Types in Relation to Chronic Pain.” Pain 160(1):S37–48. doi: 10.1097/j.pain.0000000000001529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridder De, Dirk Divya Adhia, and Vanneste Sven. 2021. “The Anatomy of Pain and Suffering in the Brain and Its Clinical Implications.” Neuroscience & Biobehavioral Reviews 130:125–46. doi: 10.1016/j.neubiorev.2021.08.013. [DOI] [PubMed] [Google Scholar]
- Rustamov Nabi, Wilson Elizabeth A., Fogarty Alexandra E., Crock Lara W., Leuthardt Eric C., and Haroutounian Simon. 2022. “Relief of Chronic Pain Associated with Increase in Midline Frontal Theta Power.” PAIN Reports 7(6):e1040. doi: 10.1097/PR9.0000000000001040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiedt-Fehr Christina, Mathes Birgit, and Basar-Eroglu Canan. 2009. “Alpha Brain Oscillations and Inhibitory Control.” Journal of Psychophysiology 23(4):208–15. doi: 10.1027/0269-8803.23.4.208. [DOI] [Google Scholar]
- Simis Marcel, Imamura Marta, Pacheco-Barrios Kevin, Marduy Anna, de Melo Paulo S., Mendes Augusto J., Teixeira Paulo E. P., Battistella Linamara, and Fregni Felipe. 2022. “EEG Theta and Beta Bands as Brain Oscillations for Different Knee Osteoarthritis Phenotypes According to Disease Severity.” Scientific Reports 12(1):1480. doi: 10.1038/s41598-022-04957-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sufianov AA, Shapkin AG, Sufianova GZ, Elishev VG, Barashin DA, Berdichevskii VB, and Churkin SV 2014. “Functional and Metabolic Changes in the Brain in Neuropathic Pain Syndrome against the Background of Chronic Epidural Electrostimulation of the Spinal Cord.” Bulletin of Experimental Biology and Medicine 157(4):462–65. doi: 10.1007/s10517-014-2591-0. [DOI] [PubMed] [Google Scholar]
- Dinh Ta, Son Moritz M. Nickel, Tiemann Laura, May Elisabeth S., Heitmann Henrik, Hohn Vanessa D., Edenharter Günther, Utpadel-Fischler Daniel, Tölle Thomas R., Sauseng Paul, Gross Joachim, and Ploner Markus. 2019. “Brain Dysfunction in Chronic Pain Patients Assessed by Resting-State Electroencephalography.” Pain 160(12):2751–65. doi: 10.1097/j.pain.0000000000001666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taesler Philipp, and Rose Michael. 2016. “Prestimulus Theta Oscillations and Connectivity Modulate Pain Perception.” The Journal of Neuroscience : The Official Journal of the Society for Neuroscience 36(18):5026–33. doi: 10.1523/JNEUROSCI.3325-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW, Hansson P, Hughes R, Nurmikko T, and Serra J. 2008. “Neuropathic Pain.” Neurology 70(18):1630–35. doi: 10.1212/01.wnl.0000282763.29778.59. [DOI] [PubMed] [Google Scholar]
- Zebhauser Paul Theo, Hohn Vanessa D., and Ploner Markus. 2023. “Resting-State Electroencephalography and Magnetoencephalography as Biomarkers of Chronic Pain: A Systematic Review.” Pain 164(6):1200–1221. doi: 10.1097/j.pain.0000000000002825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Shu, Lithfous Ségolène, Després Olivier, Pebayle Thierry, Bi Xiaoying, and Dufour André. 2020. “Involvement of Frontal Functions in Pain Tolerance in Aging: Evidence From Neuropsychological Assessments and Gamma-Band Oscillations.” Frontiers in Aging Neuroscience 12:131. doi: 10.3389/fnagi.2020.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zis Panagiotis, Liampas Andreas, Artemiadis Artemios, Tsalamandris Gabriela, Neophytou Panagiota, Unwin Zoe, Kimiskidis Vasilios K., Hadjigeorgiou Georgios M., Varrassi Giustino, Zhao Yifan, and Sarrigiannis Ptolemaios Georgios. 2022. “EEG Recordings as Biomarkers of Pain Perception: Where Do We Stand and Where to Go?” Pain and Therapy 11(2):369–80. doi: 10.1007/s40122-022-00372-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.