Abstract
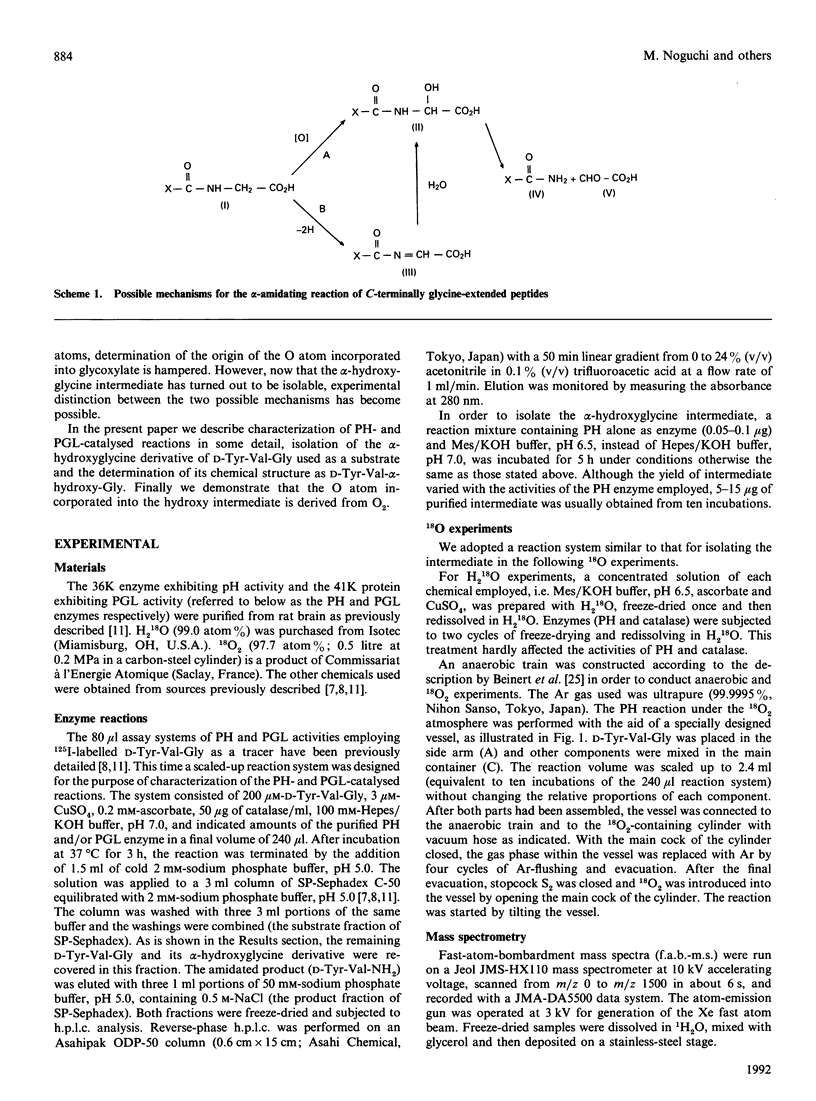
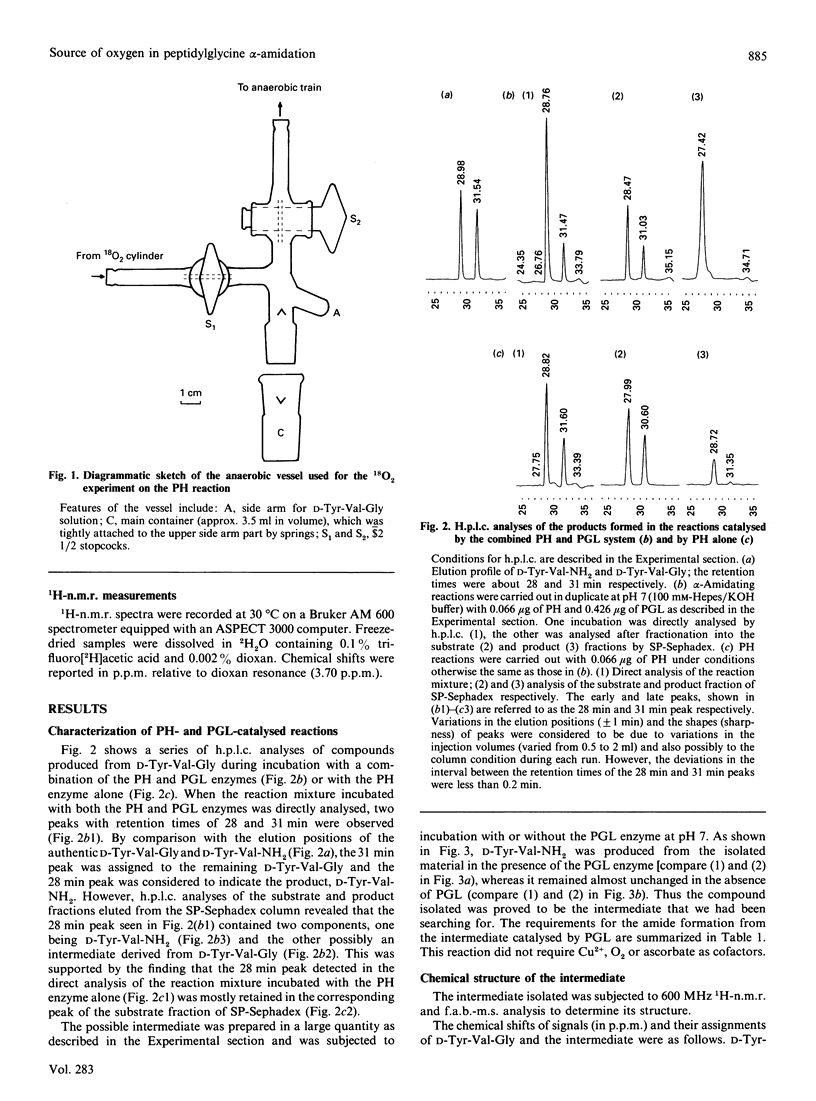
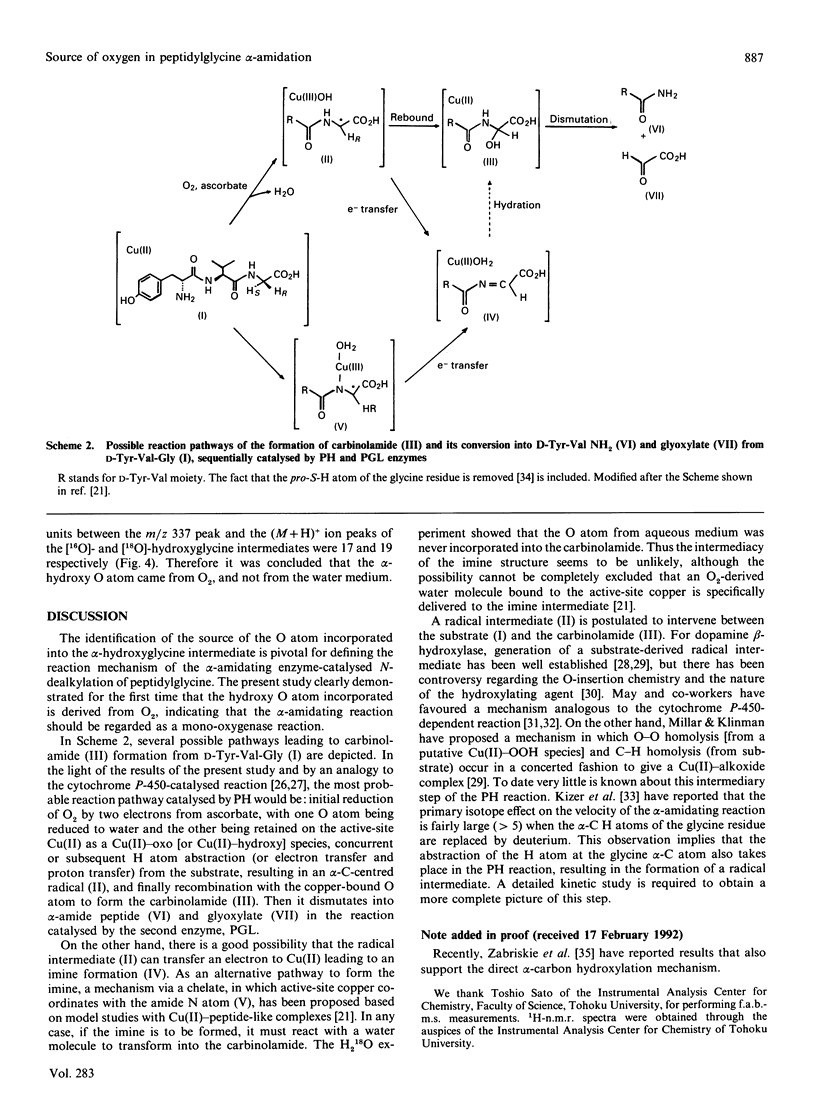
Peptidylglycine alpha-amidating activity catalyses the oxidation of a C-terminally glycine-extended peptide to a desglycine alpha-amidated peptide at the expense of ascorbate and O2 in the presence of Cu2+. The reaction involves oxidative N-dealkylation within the terminal glycine residue, with retention of the glycine N atom and release of the remainder as glyoxylate. Recent studies by us and others have revealed that the reaction consists of two steps via a carbinolamide as an intermediate (peptidyl alpha-hydroxyglycine), and also that two separate enzymes derived from a common precursor protein catalyse these steps, formation of the carbinolamide and its conversion into alpha-amide and glyoxylate. As for the mechanism of carbinolamide formation, two distinct pathways can be considered: direct mono-oxygenation at the glycine alpha-C atom and dehydrogenation leading to an imine followed by hydration. To draw a distinction between them, we carried out the reaction with D-Tyr-Val-Gly as the substrate either in the H2(18)O-enriched medium or under an atmosphere of 18O2, and isolated the alpha-hydroxylglycine intermediate. The fast-atom-bombardment mass-spectral analysis demonstrated that the hydroxy O atom comes from O2, but not from H2O, indicating that the alpha-hydroxylation should be a monooxygenase reaction.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beinert H., Orme-Johnson W. H., Palmer G. Special techniques for the preparation of samples for low-temperature EPR spectroscopy. Methods Enzymol. 1978;54:111–132. doi: 10.1016/s0076-6879(78)54013-5. [DOI] [PubMed] [Google Scholar]
- Bradbury A. F., Finnie M. D., Smyth D. G. Mechanism of C-terminal amide formation by pituitary enzymes. Nature. 1982 Aug 12;298(5875):686–688. doi: 10.1038/298686a0. [DOI] [PubMed] [Google Scholar]
- Bradbury A. F., Smyth D. G. Enzyme-catalysed peptide amidation. Isolation of a stable intermediate formed by reaction of the amidating enzyme with an imino acid. Eur J Biochem. 1987 Dec 15;169(3):579–584. doi: 10.1111/j.1432-1033.1987.tb13648.x. [DOI] [PubMed] [Google Scholar]
- Bradbury A. F., Smyth D. G. Peptide amidation. Trends Biochem Sci. 1991 Mar;16(3):112–115. doi: 10.1016/0968-0004(91)90044-v. [DOI] [PubMed] [Google Scholar]
- Bradbury A. F., Smyth D. G. Substrate specificity of an amidating enzyme in porcine pituitary. Biochem Biophys Res Commun. 1983 Apr 29;112(2):372–377. doi: 10.1016/0006-291x(83)91473-0. [DOI] [PubMed] [Google Scholar]
- Eipper B. A., Mains R. E., Glembotski C. C. Identification in pituitary tissue of a peptide alpha-amidation activity that acts on glycine-extended peptides and requires molecular oxygen, copper, and ascorbic acid. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5144–5148. doi: 10.1073/pnas.80.16.5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eipper B. A., Myers A. C., Mains R. E. Peptidyl-glycine alpha-amidation activity in tissues and serum of the adult rat. Endocrinology. 1985 Jun;116(6):2497–2504. doi: 10.1210/endo-116-6-2497. [DOI] [PubMed] [Google Scholar]
- Eipper B. A., Perkins S. N., Husten E. J., Johnson R. C., Keutmann H. T., Mains R. E. Peptidyl-alpha-hydroxyglycine alpha-amidating lyase. Purification, characterization, and expression. J Biol Chem. 1991 Apr 25;266(12):7827–7833. [PubMed] [Google Scholar]
- Emeson R. B. Hypothalamic peptidyl-glycine alpha-amidating monooxygenase: preliminary characterization. J Neurosci. 1984 Oct;4(10):2604–2613. doi: 10.1523/JNEUROSCI.04-10-02604.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick P. F., Flory D. R., Jr, Villafranca J. J. 3-Phenylpropenes as mechanism-based inhibitors of dopamine beta-hydroxylase: evidence for a radical mechanism. Biochemistry. 1985 Apr 23;24(9):2108–2114. doi: 10.1021/bi00330a003. [DOI] [PubMed] [Google Scholar]
- Johnson-Flanagan A. M., Huiwen Z., Thiagarajah M. R., Saini H. S. Role of Abscisic Acid in the Induction of Freezing Tolerance in Brassica napus Suspension-Cultured Cells. Plant Physiol. 1991 Apr;95(4):1044–1048. doi: 10.1104/pp.95.4.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato I., Yonekura H., Tajima M., Yanagi M., Yamamoto H., Okamoto H. Two enzymes concerned in peptide hormone alpha-amidation are synthesized from a single mRNA. Biochem Biophys Res Commun. 1990 Oct 15;172(1):197–203. doi: 10.1016/s0006-291x(05)80193-7. [DOI] [PubMed] [Google Scholar]
- Katopodis A. G., Ping D. S., Smith C. E., May S. W. Functional and structural characterization of peptidylamidoglycolate lyase, the enzyme catalyzing the second step in peptide amidation. Biochemistry. 1991 Jun 25;30(25):6189–6194. doi: 10.1021/bi00239a016. [DOI] [PubMed] [Google Scholar]
- Katopodis A. G., Ping D., May S. W. A novel enzyme from bovine neurointermediate pituitary catalyzes dealkylation of alpha-hydroxyglycine derivatives, thereby functioning sequentially with peptidylglycine alpha-amidating monooxygenase in peptide amidation. Biochemistry. 1990 Jul 3;29(26):6115–6120. doi: 10.1021/bi00478a001. [DOI] [PubMed] [Google Scholar]
- Kedderis G. L., Hollenberg P. F. Peroxidase-catalyzed N-demethylation reactions. Substrate deuterium isotope effects. J Biol Chem. 1984 Mar 25;259(6):3663–3668. [PubMed] [Google Scholar]
- Kedderis G. L., Rickert D. E., Pandey R. N., Hollenberg P. F. 18O studies of the peroxidase-catalyzed oxidation of N-methylcarbazole. Mechanisms of carbinolamine and carboxaldehyde formation. J Biol Chem. 1986 Dec 5;261(34):15910–15914. [PubMed] [Google Scholar]
- Kizer J. S., Bateman R. C., Jr, Miller C. R., Humm J., Busby W. H., Jr, Youngblood W. W. Purification and characterization of a peptidyl glycine monooxygenase from porcine pituitary. Endocrinology. 1986 Jun;118(6):2262–2267. doi: 10.1210/endo-118-6-2262. [DOI] [PubMed] [Google Scholar]
- Miller S. M., Klinman J. P. Secondary isotope effects and structure-reactivity correlations in the dopamine beta-monooxygenase reaction: evidence for a chemical mechanism. Biochemistry. 1985 Apr 23;24(9):2114–2127. doi: 10.1021/bi00330a004. [DOI] [PubMed] [Google Scholar]
- Noguchi M., Takahashi K., Okamoto H. Characterization of peptidylglycine alpha-amidating activities in rat pituitary, brain and small intestine using glycine-extended C-terminal analogues of vasoactive intestinal polypeptide as substrate. Tohoku J Exp Med. 1988 Oct;156(2):191–207. doi: 10.1620/tjem.156.191. [DOI] [PubMed] [Google Scholar]
- Noguchi M., Takahashi K., Okamoto H. Rat peptidylglycine alpha-amidating enzyme: the relation between activities at neutral and alkaline pH Values. Arch Biochem Biophys. 1989 Dec;275(2):505–513. doi: 10.1016/0003-9861(89)90397-4. [DOI] [PubMed] [Google Scholar]
- Perkins S. N., Husten E. J., Eipper B. A. The 108-kDA peptidylglycine alpha-amidating monooxygenase precursor contains two separable enzymatic activities involved in peptide amidation. Biochem Biophys Res Commun. 1990 Sep 28;171(3):926–932. doi: 10.1016/0006-291x(90)90772-f. [DOI] [PubMed] [Google Scholar]
- Perkins S. N., Husten E. J., Mains R. E., Eipper B. A. pH-dependent stimulation of peptidylglycine alpha-amidating monooxygenase activity by a granule-associated factor. Endocrinology. 1990 Dec;127(6):2771–2778. doi: 10.1210/endo-127-6-2771. [DOI] [PubMed] [Google Scholar]
- Stewart L. C., Klinman J. P. Dopamine beta-hydroxylase of adrenal chromaffin granules: structure and function. Annu Rev Biochem. 1988;57:551–592. doi: 10.1146/annurev.bi.57.070188.003003. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Shimoi H., Iwasaki Y., Kawahara T., Matsuura Y., Nishikawa Y. Elucidation of amidating reaction mechanism by frog amidating enzyme, peptidylglycine alpha-hydroxylating monooxygenase, expressed in insect cell culture. EMBO J. 1990 Dec;9(13):4259–4265. doi: 10.1002/j.1460-2075.1990.tb07874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima M., Iida T., Yoshida S., Komatsu K., Namba R., Yanagi M., Noguchi M., Okamoto H. The reaction product of peptidylglycine alpha-amidating enzyme is a hydroxyl derivative at alpha-carbon of the carboxyl-terminal glycine. J Biol Chem. 1990 Jun 15;265(17):9602–9605. [PubMed] [Google Scholar]
- Takahashi K., Okamoto H., Seino H., Noguchi M. Peptidylglycine alpha-amidating reaction: evidence for a two-step mechanism involving a stable intermediate at neutral pH. Biochem Biophys Res Commun. 1990 Jun 15;169(2):524–530. doi: 10.1016/0006-291x(90)90362-q. [DOI] [PubMed] [Google Scholar]
- White R. E., Coon M. J. Oxygen activation by cytochrome P-450. Annu Rev Biochem. 1980;49:315–356. doi: 10.1146/annurev.bi.49.070180.001531. [DOI] [PubMed] [Google Scholar]


