Abstract
Citrin deficiency (CD) is a hereditary disorder caused by SLC25A13 mutations that manifests as neonatal intrahepatic cholestasis caused by CD (NICCD), failure to thrive and dyslipidemia caused by CD (FTTDCD), and adult-onset type 2 citrullinemia (CTLN2). Citrin, an aspartate-glutamate carrier primarily expressed in the liver, is a component of the malate-aspartate shuttle, which is essential for glycolysis. Citrin-deficient hepatocytes have primary defects in glycolysis and de novo lipogenesis and exhibit secondarily downregulated PPARα, leading to impaired β-oxidation. They are unable to utilize glucose and free fatty acids as energy sources, resulting in energy deficiencies. Medium-chain triglyceride (MCT) supplements are effective for treating CD by providing energy to hepatocytes, increasing lipogenesis, and activating the malate-citrate shuttle. However, patients with CD often exhibit growth impairment and irreversible brain and/or liver damage. To improve the quality of life and prevent irreversible damage, MCT supplementation with a diet containing minimal carbohydrates is recommended promptly after the diagnosis.
Keywords: citrin, SLC25A13, neonatal intrahepatic cholestasis caused by citrin deficiency, adult-onset type 2 citrullinemia, medium-chain triglycerides
Introduction
Two clinical and genetic forms of citrullinemia have been identified. Type I citrullinemia (CTLN1) (OMIM 215700) is caused by a deficiency in argininosuccinate synthase 1 (ASS1) in the urea cycle, whereas adult-onset type 2 citrullinemia (CTLN2) (OMIM 603417) is caused by deficiency of citrin, an aspartate-glutamate carrier in the inner mitochondrial membrane (1,2). CTLN1 and CTLN2 are caused by the pathogenic variants of ASS1 and SLC25A13, respectively. Citrin deficiency (CD) is prevalent in Japan and was first reported by Miyakoshi et al. (3) in 1968, with its causative gene (SLC25A13) first identified by Kobayashi et al. (1) in 1999. Elucidation of the etiology has revealed that CD presents with age-dependent symptoms, manifesting as neonatal intrahepatic cholestasis caused by CD (NICCD) (OMIM 605814), failure to thrive and dyslipidemia caused by CD (FTTDCD), and CTLN2 (2).
We herein review the pathogenesis and treatment of CD and the prevention of irreversible damage due to CD.
1. Citrin and Its Encoding Gene
Citrin is an aspartate-glutamate carrier in the inner mitochondrial membrane consisting of 675 amino acid residues with Ca2+-binding domains and has the characteristic structure of a calcium-binding mitochondrial solute-carrier protein. Citrin is mainly expressed in the liver and is a component of the malate-aspartate shuttle, which transports NADH produced by glycolysis from the cytosol to the mitochondria and regenerates cytosolic NAD+ (Fig. 1) (1,4). As a component of this shuttle, citrin is indispensable for hepatic glycolysis, de novo lipogenesis, and energy metabolism (5). Citrin binds to fluxed Ca2+, enhances aspartate-glutamate carrier activity, increases ATP production, and protects hepatocytes from stress (4,6).
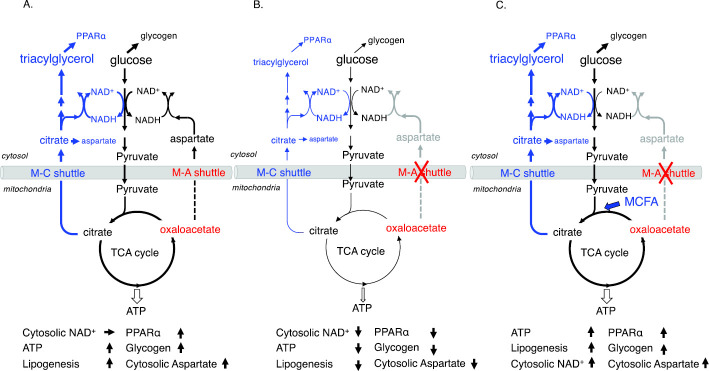
Figure 1.
Glycolysis and lipogenesis in the liver. Acetyl-CoA: acetyl coenzyme A, KG: ketoglutarate, ChREBP: carbohydrate-responsive element-binding protein, CiC: citrate carrier, NADH+: nicotinamide adenine dinucleotide hydrogen, NAD+: nicotinamide adenine dinucleotide-oxidized, OAA: oxaloacetate, OGC: 2-oxoglutarate carrier, PyC: pyruvate carrier, SREBP-1: sterol regulatory element-binding protein-1, TCA: tricarboxylic acid
Citrin is encoded by SLC25A13, which is 160 kb in size, consists of 18 exons, and is located on chromosome 7q21.3 (1,2,4). Citrin is expressed in the liver, kidneys, and heart. Aralar is an isoprotein of citrin that is mainly expressed in the brain, muscles, and heart. Citrin and aralar complement each other as aspartate-glutamate carriers. Due to their differential expression, citrin and aralar are essential components of the malate-aspartate shuttle in the liver and brain, respectively.
2. Pathogenic SLC25A13 Variants and the Genotype-phenotype Relationship
CD is a hereditary disease caused by SLC25A13 pathogenic variants, and patients are either homozygous or compound heterozygous for these variants. More than 100 citrin pathogenic variants have been reported, including frameshift, nonsense, and missense mutations (7). CD is prevalent in East Asian countries, including Japan, and frequent mutations have been reported in this population. The carrier rates are estimated to be 1:42 and 1:65 in northern and southern Japan, respectively (8,9). Pathogenic variants act in a loss-of-function manner and do not exhibit a genotype-phenotype relationship. Other genetic and environmental factors are also associated with this phenotype.
3. Energy and Glucose Metabolism in the Liver
The liver consumes approximately 20% of the daily caloric requirements. During fasting, hepatocytes utilize free fatty acids that are mobilized from adipose tissues as an energy source. During the postprandial hyperglycemic period, hepatocytes take up glucose via glucose transporter 2 (GLUT2) and use most of it for glycogenesis and some for de novo lipogenesis, amino acid synthesis, and as an energy source (10). Hepatic de novo lipogenesis regulates energy metabolism through the regulation of peroxisome proliferator-activated α (PPARα); its promotion upregulates PPARα, and its suppression downregulates PPARα (Fig. 2A) (11-13). PPARα promotes the uptake, utilization, and catabolism of fatty acids by upregulating the genes involved in fatty acid transport, fatty acid binding and activation, and fatty acid β-oxidation. De novo lipogenesis also plays an important role in lipid supply during growth spurt periods, such as fetal development, infancy, and adolescence (14-16).
Figure 2.
A: Glucose metabolism in control livers. B: Glucose metabolism in citrin-deficient livers. C: Metabolic changes in citrin-deficient livers following medium-chain triglyceride (MCT) supplementation. Black lines represent glycolysis, glycogenesis, and the TCA cycle, whereas blue lines represent de novo lipogenesis. ATP: adenosine triphosphate, M-A shuttle: malate-aspartate shuttle, M-C shuttle: malate-citrate shuttle, MCFA: medium-chain free fatty acids, PPARα: peroxisome proliferator-activated receptor α, TCA: tricarboxylic acid
4. Energy and Glucose Metabolism in the Livers of Patients with CD and Changes after Medium-chain Triglyceride Supplementation
Individuals with CD have a primary defect in hepatic glycolysis and de novo lipogenesis due to impairment of the malate-aspartate shuttle and show secondary downregulation of PPARα due to impaired de novo lipogenesis and/or endoplasmic reticulum (ER) stress (Fig. 3) (12,17-20). Therefore, hepatocytes of individuals with CD are unable to utilize glucose and free fatty acids for energy, resulting in energy deficiencies (5).
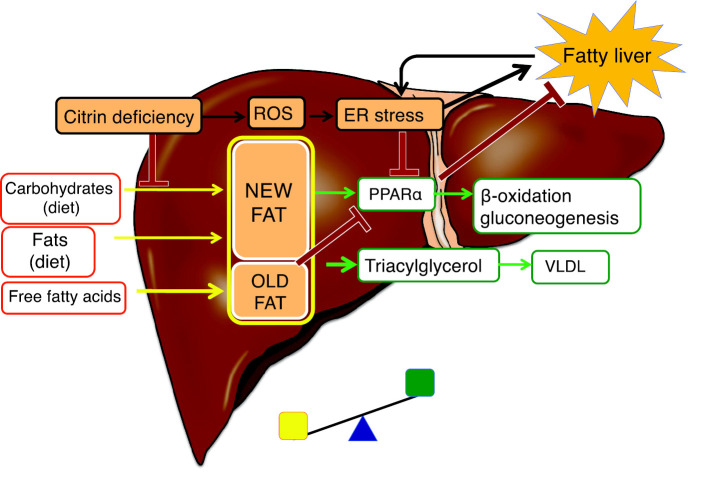
Figure 3.
Fatty liver in citrin deficiency. Hepatocytes synthesize new fat from dietary carbohydrates and fats and synthesize old fat from incorporated plasma-free fatty acids. Fat accumulates when the fat synthesis and uptake exceeds β-oxidation and VLDL levels (exported fat). New fat upregulates PPARα, whereas old fat downregulates PPARα. PPARα is downregulated by impaired de novo lipogenesis and/or ER stress in the liver of adult-onset type 2 citrullinemia (CTLN2), leading to a fatty liver. ER: endoplasmic reticulum, PPARα: peroxisome proliferator-activated receptor α, ROS: reactive oxygen species, VLDL: very-low-density lipoprotein
In individuals with CD, hepatic glycogenesis, gluconeogenesis, and ketogenesis are impaired owing to a lack of hepatic energy and cytosolic NAD+ and the downregulation of PPARα. Children often fail to consume minimal carbohydrates due to food preferences or illnesses, resulting in hypoketotic hypoglycemia.
As shown in Fig. 2B, cytosolic NAD+, ATP, glycogenesis, lipogenesis, and cytosolic aspartate levels were predicted to be reduced in citrin-deficient hepatocytes (5,18-20).
Medium-chain triglycerides (MCTs) are absorbed without bile acids, are primarily metabolized in hepatocytes, provide energy and acetyl-CoA to hepatocytes, enhance tricarboxylic acid (TCA) cycle activity, increase glycogenesis, and stimulate de novo lipogenesis, resulting in upregulated PPARα and increased cytosolic aspartate and NAD+ levels through the malate-citrate shuttle (Fig. 2C). Increased NAD+ levels promote glycolysis, and MCT administration corrects most of the metabolic abnormalities associated with CD in the liver (5,18-20).
5. Pathogeneses of Liver Dysfunction
5.1. NICCD
Individuals with CD have a relatively low birth weight and short body length (2,5,16). Approximately 40% of patients are identified through newborn mass screening tests, which reveal elevated blood galactose, methionine, and phenylalanine levels. Patients also present with intrahepatic cholestasis with or without cataracts (2). Laboratory findings indicate increased levels of cholestatic markers, α-fetoprotein, galactose, and plasma amino acids including citrulline. Liver biopsies reveal cholestasis and steatosis. In patients presenting with these symptoms, the diagnosis is confirmed by a SLC25A13 genetic analysis. During the fetal period, the energy source for hepatocytes is amino acids, which are converted to free fatty acids and glucose after birth. In addition, lactose (a disaccharide of glucose and galactose) is a major carbohydrate in milk, and galactose metabolism is inhibited at the galactose epimerase step because of increased cytosolic NADH, resulting in the accumulation of toxic metabolites (21). Notably, the demand for lipogenesis is high in the first six months of life (16,21). These factors may be associated with NICCD pathogenesis.
5.2. FTTDCD
Most patients with CD experience an “apparently healthy” period (from post-NICCD to before the onset of CTLN2); however, some patients report recurrent hypoglycemia, growth impairment, fatigability, hyperlipidemia, and pancreatitis. These patients are diagnosed with FTTDCD (2). In Japan, patients with FTTDCD are rare, probably because of the recommended low-carbohydrate diet and/or MCT supplementation. These clinical abnormalities may be caused by a continuous energy deficit and exposure to oxidative and/or ER stress in hepatocytes.
5.3. CTLN2
The onset of CTLN2 is associated with genetic (including variants other than SLC25A13) and environmental factors. Hyperammonemic encephalopathy develops in approximately 5% of citrin-deficient individuals 10-70 years old (2). A preponderance in men and individuals with a lean body is characteristic, and approximately 10% of patients experience complications of hyperlipidemia, pancreatitis, and hepatocellular carcinoma. The onset is often associated with surgery (fasting), alcohol consumption, diabetes, and medication use. Patients usually present with nocturnal hyperammonemic encephalopathy. With regard to the diagnosis, some patients have a history of NICCD, and their food preferences provide useful information. The absence of increased plasma glutamine concentrations despite hyperammonemia is also a characteristic symptom associated with CTLN2 (20). Liver biopsies and imaging studies have revealed fatty liver in these patients; however, a definitive diagnosis is dependent on a genetic analysis of SLC25A13. The development of CTLN2 may be related to long-term energy deficits and oxidative and ER stress in hepatocytes (5).
5.4. Fatty liver
Fatty liver has been observed in patients with NICCD and CTLN2. Komatsu et al. (17) observed a marked suppression of the mRNA-encoding enzymes/proteins involved in fatty acid oxidation and the downregulation of PPARα in the liver of patients with CTLN2, most likely due to ER stress. PPARα is upregulated by new fat (synthesized from dietary carbohydrates and fats) in the liver and downregulated by old fat (mobilized from adipose tissue) (12). Patients present with defects in de novo lipogenesis, leading to PPARα downregulation (5,18). Lipid deposition in the liver, in turn, affects sensitivity to insulin and causes ER stress. Based on these findings, it is suggested that fatty liver is mainly caused by PPARα downregulation (Fig. 3). Furthermore, early treatment with MCT supplementation improves fatty liver in CTLN2 patients, supporting the mechanism by which PPARα is downregulated (5,19,20). It has been speculated that malate-citrate shuttle activity is increased to complement the malate-aspartate shuttle defect and promote de novo lipogenesis, leading to a fatty liver (22). However, this is unlikely, as de novo lipogenesis requires energy, and citrin-deficient hepatocytes are expected to be in a low-energy state.
5.5. Citrullinemia
Citrulline is metabolized by ASS1, which requires aspartate and ATP. In the liver, citrin is the only transport system of aspartate from the mitochondria to the cytosol, and its deficiency has been predicted to cause aspartate deficiency, leading to citrullinemia (23). However, citrullinemia has only been observed during periods of NICCD or CTLN2, indicating that aspartate required for the ASS1 reaction can be supplied, even in the citrin-deficient hepatocytes. In patients with NICCD, the plasma concentrations of the constituent amino acids of the urea cycle, including citrulline, were elevated. However, hepatic ASS1 enzyme activity was normal (24), and MCT supplementation normalized the concentrations of these amino acids within a few days (21). These findings suggest that citrullinemia in NICCD is most likely caused by aspartate and/or ATP deficiency for ASS1 reaction. MCT supplementation increases ATP production, de novo lipogenesis, and citrate transport from the mitochondria to the cytosol via the malate-citrate shuttle. Cytosolic citrate can be metabolized to oxaloacetate by ATP-citrate lyase and further metabolized to aspartate by aspartate aminotransferase (Fig. 1, , 2C, 4). In addition to the malate-aspartate shuttle, this metabolic pathway can also supply aspartate for ASS1 reaction in the cytosol.
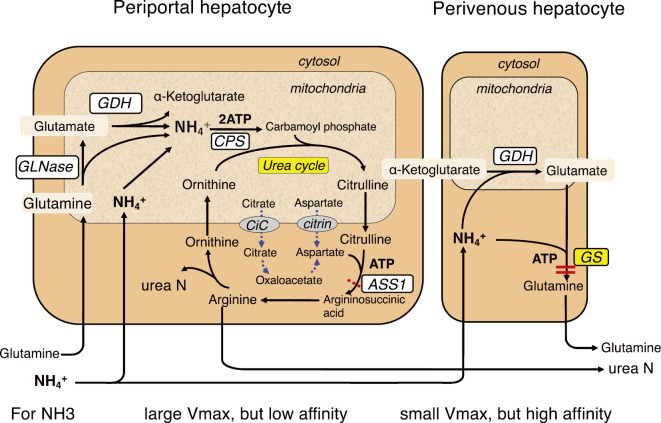
Figure 4.
Ammonia detoxification system in the liver. Two ammonia detoxification systems exist in the liver. Ureagenesis in periportal hepatocytes has a low affinity for ammonia but a high ammonia detoxification capacity. Glutamine synthesis in perivenous hepatocytes has a low detoxification capacity but a high affinity for ammonia. ASS1: argininosuccinate synthetase 1, CiC: citrate carrier, CPS: carbamoylphosphate synthetase, GDH: glutamate dehydrogenase, GLNase: glutaminase, GS: glutamine synthetase
In contrast, while hyperammonemia can be normalized with MCT supplementation in most patients with CTLN2, citrullinemia cannot be normalized, except for in two patients who were treated early after the onset (Patients 2 and 3 in Table 1) (5,19,20,25-29). Saheki et al. (2,30) reported that hepatic ASS1 activity was reduced in most patients with CTLN2 due to a decrease in the enzyme protein. These findings suggest that the ASS1 reaction is differently impaired in patients with NICCD and CTLN2 owing to substrate deficiencies and a reduction in the ASS1 enzyme protein, respectively.
Table 1.
Clinical Data of Patients with CTLN2 Treated with MCT.
| Gender | Age at onset | Start of Tx after onset | Plasma Cit (mM) before/after Tx (n: 17.9-48.0) | Plasma Gln (mM) before/after Tx (n: 422.1-739.8) | Recurrence of HE | Prognosis | Complication | |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 37y | 16y | 74.9/60.2 | 387.7/472.9 | No | Good | (ref. 21) |
| 2 | M | 53y | 2m | 102.9/45.7 | 679.4/711.1 | No | Fair | (ref. 21) |
| 3 | M | 62y | 1m | 131.4/33.3 | 360.6/558.0 | No | Fair | Hepatoma (surgically removed) (ref. 21) |
| 4 | M | 38y | 13y | 462.0/612.9 | 450.7/530.8 | Yes | Improved | Schizophrenia (ref. 21) |
| 5 | M | 41y | ? | 451.4/133.2 | 570.9/551.6 | Yes | Improved | Irregular intake of MCT (ref. 21) |
| 6 | F | 62y | 9m | 486.4/228.2 | 473.9/762.8 | No | Good | Lung cancer (surgically removed) (ref. 22) |
| 7 | F | 48y | 4y | 839.0/515.5 | 448.1/535.2 | Yes | Good | Diabetes (insulin Tx) (ref. 22) |
| 8 | F | 60y | 7m | 407.4/211.9 | 466.8/650.3 | No | Good | Liver transplantation (ref. 22) |
| 9 | M | 67y | 3m | 541.2/157.1 | 320.4/565.3 | No | Good | (ref. 22) |
| 10 | M | 54y | 5m | 384.3/557.0 | 580.7/624.2 | No | Good | (ref. 22) |
| 11 | M | 61y | 12m | 197.8/54.2 | 357.8/648.0 | No | Good | Diabetes (insulin Tx), Pancreatic cancer (ref. 25) |
| 12 | M | 33y | 20m | 235.4/na | 427.9/na | No | Good | Short bowel (ref. 26) |
| 13 | F | 49y | 3m | 348.4/421.5 | na/571.3 | No | Good | Mental retardation, Epilepsy (ref. 27) |
| 14 | F | 44y | 6m | 205.2/105.3 | na/na | No | Good | Mental retardation, Epilepsy (ref. 28) |
| 15 | M | 29y | ? | 715.5/na | na/na | No | Good | No HE after regular intake of MCT (ref. 29) |
Hyperammonemia improved with MCT supplementation therapy in all patients, but citrullinemia persisted, except in Patients 2 and 3. Before treatment, plasma glutamine levels did not increase above the normal range in all patients despite hyperammonemia, rather decreased in several patients. Cit: citrulline, Gln: glutamine, HE: hyperammonemic encephalopathy, n: normal range, na: not available, Tx: treatment, m: month, y: year
5.6. Hyperammonemia in CTLN2
The liver has two ammonia-detoxification systems: ureagenesis and glutamine synthesis (Fig. 4) (31). Ureagenesis in periportal hepatocytes has a low affinity for ammonia but a high ammonia detoxification capacity. Glutamine synthesis in perivenous hepatocytes has a high affinity for ammonia and maintains physiologically low ammonia concentrations. Impairment of either system can cause hyperammonemia. Most patients with CTLN2 exhibit reduced hepatic ASS1 activity; however, plasma citrulline levels and residual ASS1 activity in patients with CTLN2 are similar to those in asymptomatic benign CTLN1 patients (2,31-33). In addition, some patients with CTLN2 display normal ASS1 activity (30), and citrullinemia persists in most patients, even after treatment normalizes blood ammonia (Table 1). This suggests that hepatic ASS1 impairment in patients with CTLN2 can cause citrullinemia but is not sufficient to cause hyperammonemia. With regard to glutamine synthesis, despite hyperammonemia, plasma glutamine concentrations did not increase above normal levels in all patients but rather decreased in some patients (Table 1). This suggests that the glutamine synthesis system did not function in ammonia-detoxification in the patient's liver. In contrast, hepatic glutamine synthetase (GS) activity was not reduced in these CTLN2 patients; its activity was 11.0±2.2 nmol/min/mg protein (n=4) vs. control 12.6±3.7 (n=7) (p=0.2919) (unpublished data) (5,20). As shown in Table 1, MCT supplementation improved hyperammonemia and normalized plasma glutamine concentrations in all patients (19,20). These findings indicate that hyperammonemia is not primarily caused by a defect in ASS1 but rather by a defect in GS due to a substrate (glutamate) and/or ATP deficiency.
5.7. Hepatocyte maturation arrest and zoning impairment
Serum α-fetoprotein levels have been reported to be markedly high in normal-term infants and decrease with liver maturation in the first six months of life. Increased serum α-fetoprotein levels are a hallmark of NICCD (2) and can be steadily normalized through MCT supplementation with notable clinical improvements (21). The energy source of the fetal liver is amino acids, and glutamate dehydrogenase activity in the rat liver is elevated by approximately three-fold during the perinatal period compared to that in adults (34). Glutamate is actively synthesized in the developing rat liver and transaminated to other nonessential amino acids for protein synthesis. Notably, MCT supplementation in patients with NICCD markedly increased plasma glutamate concentrations from 51.3±18.8 μmol/L (n=5) (normal range 12.6-62.5) before administration to 168.3±92.0 μmol/L (p=0.012) at 2-3 weeks post-supplementation (unpublished data). Plasma glutamate concentrations were normalized with improvements in the liver function and serum α-fetoprotein levels. Increased serum α-fetoprotein levels have also been reported in patients with mitochondrial hepatopathy (35). These findings suggest that energy deficiency in hepatocytes is associated with the arrest of liver maturation.
In patients with CTLN2, changes in ASS1 and GS expression were observed in the liver (5,20,30). Metabolic zoning occurs in the liver, with periportal hepatocytes playing a critical role in glycogenesis, urea production, and fatty acid oxidation, whereas pericentral hepatocytes are involved in glycolysis, glutamine synthesis, and lipogenesis. ASS1 was shown to be ubiquitously expressed in the hepatocytes of control livers, with a slightly increased expression in periportal hepatocytes, whereas ASS1-expressing hepatocytes were reduced in patients with CTLN2 (5,20,36). In contrast, GS-positive hepatocytes in control livers were restricted to around the central veins. However, in patients with CTLN2, their distribution was not restricted to the hepatocytes around the central veins but more widespread (5,19). Early MCT treatment normalized the distribution of ASS1- and GS-positive hepatocytes (5,20). These findings suggest that liver zonation is impaired in patients with CTLN2, most likely because of energy deficiency, leading to irreversible liver damage. GS expression is upregulated by Wnt/β-catenin signaling, and abnormal expression of the Wnt/β-catenin pathway may be associated with complications in hepatocellular carcinoma (37).
6. Pathophysiology of Various Symptoms
6.1. Growth failure
In addition to controlling energy metabolism, hepatic de novo lipogenesis also plays an important role in lipid supply during the fetal (third trimester), infant (first six months of life), and adolescent growth spurt periods. Growth impairment during these periods is a complication of CD (16).
6.2. Food preferences
Most individuals with CD prefer high-protein and high-fat foods, such as bean products, eggs, fish, and meat. In contrast, they tend to dislike carbohydrate-rich foods, such as cereals, sweets, and cooked rice. Citrin-deficient hepatocytes are unable to generate ATP from carbohydrates; however, proteins (amino acids) and fats (fatty acids) produce energy through pathways that do not require NAD+ (5,18). The liver is known to have a metabolic-sensing function (38), and patients are likely to prefer foods that provide energy to hepatocytes. When considering the relationship between food preference and carbohydrate toxicity, we must also consider that carbohydrate-induced hyperglycemia induces insulin secretion and decreases free fatty acids, which are major energy sources for citrin-deficient hepatocytes (5). A decrease in hepatic energy may cause discomfort and frustration. MCT supplementation alters food preferences and promotes carbohydrate intake. Furthermore, most patients are averse to alcohol, and drinking can trigger the onset of CTLN2. Alcohol metabolism is impaired, as reactions involving alcohol and aldehyde dehydrogenases require NAD+.
6.3. Carbohydrate toxicity
The existence of carbohydrate toxicity has been inferred from reports suggesting that carbohydrate-rich diets and formulae containing lactose exacerbated CTLN2 and NICCD (39), respectively; hyperalimentation was found to cause hyperammonemic encephalopathy in a patient (40); and infusions of osmotic agents containing glycerol and fructose were lethal in CTLN2 patients (41).
Changing from a protein- and fat-rich diet to a carbohydrate-rich diet may induce CTLN2. In citrin-deficient hepatocytes, the energy deficit is exacerbated by a carbohydrate-rich diet due to the reduced dietary protein and fat available to hepatocytes. In addition, hyperglycemia induced by a carbohydrate-rich diet decreases the levels of plasma free fatty acids, which are essential energy sources for citrin-deficient hepatocytes. In NICCD, a formula containing lactose can lead to the accumulation of toxic galactose metabolites (as described in the pathogenesis of liver dysfunction).
Notably, the hepatic glucose uptake and metabolism are limited under normoglycemic conditions and increase under postprandial hyperglycemic conditions, owing to the low glucose affinity of GLUT2 (5,25). In addition, the hepatic glucose uptake through oral administration is approximately twice that achieved through intravenous administration due to portal glucose signaling (42). Hyperalimentation was reported to induce hyperammonemic encephalopathy with hyperglycemia in only one patient with CTLN2 (40). That patient was severely emaciated and likely glucose intolerant, and their condition improved when the infusion was discontinued. Due to the low glucose affinity of GLUT2 and the lack of portal glucose signaling, hyperalimentation can be safely adopted if persistent hyperglycemia is avoided and nutrients available to hepatocytes, such as lipids (formulas containing MCTs are recommended) and amino acids, are supplemented. Hyperalimentation was actually performed prior to liver transplantation in two patients with CTLN2 (43). Symptomatic hypoglycemia was treated with low-concentration glucose infusions but could be safely treated with a bolus of 10% glucose solution.
Glucose toxicity has been definitively observed in patients with CTLN2 and diabetes mellitus (25). Hyperammonemia and liver dysfunction were found to improve after the initiation of insulin therapy in addition to MCT supplementation. When hyperglycemia persists, more glucose is absorbed and metabolized through glycolysis; however, its metabolism is inhibited at the glyceraldehyde 3-phosphate dehydrogenase step, leading to the accumulation of glucose metabolites and a decrease in Pi and ATP levels in hepatocytes. These findings suggest, both theoretically and clinically, that persistent hyperglycemia is toxic and that glycemic control is critical for the treatment of patients with CD.
In contrast to glucose metabolism, intravenously administered fructose is rapidly absorbed and metabolized by hepatocytes, and a fructose load of 250 mg/kg body weight has been shown to decrease liver ATP concentrations, even in healthy adults, owing to the consumption of Pi (44). Furthermore, hepatic fructose metabolism in CD is impaired at the glyceraldehyde 3-phosphate dehydrogenase step, and osmotic infusions containing glycerol and fructose are lethal, most likely due to ATP depletion (41). However, when administered orally, fructose has a low absorption rate and is partially absorbed and metabolized in intestinal villi (45). Therefore, there is no need to severely restrict the oral intake of fructose from sweets and fruits. However, excessive sugar intake (a disaccharide of glucose and fructose) should be avoided, as fructose can saturate intestinal cell absorption and may be transported to hepatocytes. In addition, it can induce hyperglycemia, leading to insulin secretion and decreased mobilization of fatty acids from the adipose tissues.
6.4. Oxidative and ER stress-related symptoms
Enhanced oxidative stress and increased levels of serum high-density lipoprotein (HDL)-cholesterol, cholesterol, and sterol markers have been reported in children with CD (46,47). Komatsu et al. (17) reported the downregulation of PPARα in the livers of patients with CTLN2, most likely due to ER stress. Citrin-deficient hepatocytes are energy-deficient, resulting in the production of reactive oxygen species (ROS), which in turn causes ER stress. Cholesterol synthesis is controlled by sterol regulatory element-binding protein 2 (SREBP-2), which is upregulated by ER stress (48). When hepatocytes are stressed, citrin binds to fluxed Ca2+ and increases aspartate-glutamate carrier activity, thus producing more ATP to protect cells. This suggests that citrin-deficient hepatocytes may be extremely vulnerable to endogenously generated stress and lack protective responses (4,6).
7. Treatment for Each Clinical Phenotype
7.1. NICCD
Patients with and without galactosemia are generally treated with lactose-free and ordinary milk, including breast milk, respectively. The formula is prepared by mixing 100 mL of milk with 2 mL of MCT oil, which constitutes 20% of the total calories (5,21). Fat-soluble vitamins should be supplemented in cases of deficiency. MCT supplementation can steadily improve cholestasis, citrullinemia, and the general condition. The laboratory findings usually improve within a few months of treatment. Early treatment is more effective than later treatment, but MCT supplementation is recommended until patients are at least three years old in order to promote central nervous system growth and myelination (5,21).
7.2. FTTDCD
After recovery from NICCD, some patients complain of recurrent hypoglycemia, growth impairment, fatigue, hyperlipidemia, and pancreatitis (2). A protein- and fat-rich diet similar to that in patients with CTLN2 is recommended to prevent the development of CTLN2 (49). However, some patients experience recurrent hypoketotic hypoglycemia due to a reduced carbohydrate intake and impaired gluconeogenesis, glycogenosis, and ketogenesis. To prevent hypoglycemia, the recommended minimum daily carbohydrate requirements are 100 g/day for children 1 year old, 100-130 g/day for children 1-5 years old, and 130 g/day for children >5 years old (5). Low-glycemic carbohydrates may also be recommended. To prevent the development of CTLN2 and improve the quality of life, MCT supplementation with a diet containing minimal carbohydrates is recommended (Fig. 2, Table 1) (5).
7.3. CTLN2
Early MCT supplementation with a low-carbohydrate formula (protein:fat:carbohydrate energy ratio of 10-20:35-50:40-45) is recommended for the management of CTLN2. The MCT dosages for men (large adults) and women (small adults) are 45 and 30 mL, respectively, and daily doses are administered as three evenly divided portions with meals (5,18-20). As shown in Table 1, hyperammonemic encephalopathy steadily improved in all patients; however, citrullinemia and fatty liver persisted, except in two patients who were treated early (5,20,26). These results indicate that MCT should be supplemented soon after the diagnosis to prevent irreversible liver damage. Sodium pyruvate therapy has also been used, but this treatment did not prevent relapse of encephalopathy or improve the Fischer ratio or citrullinemia in a previous report (5,50).
Infusion of glycerol- and fructose-containing osmotic agents is lethal and contraindicated (41). Infusion of lactate-buffered solution should also be avoided. Persistent hyperglycemia must be avoided, and glycemic control is critical in patients with diabetes mellitus and hyperalimentation (5,25). Bezafibrate administration is undesirable because it inhibits de novo lipogenesis and induces the generation of uncoupling proteins that reduce the effects of MCT (19). In addition, hypertriglyceridemia has been shown to steadily improve with MCT supplementation. Medications may affect hepatic energy metabolism and should be administered with caution.
8. Improvement in the Quality of Life and Prevention of Irreversible Damage
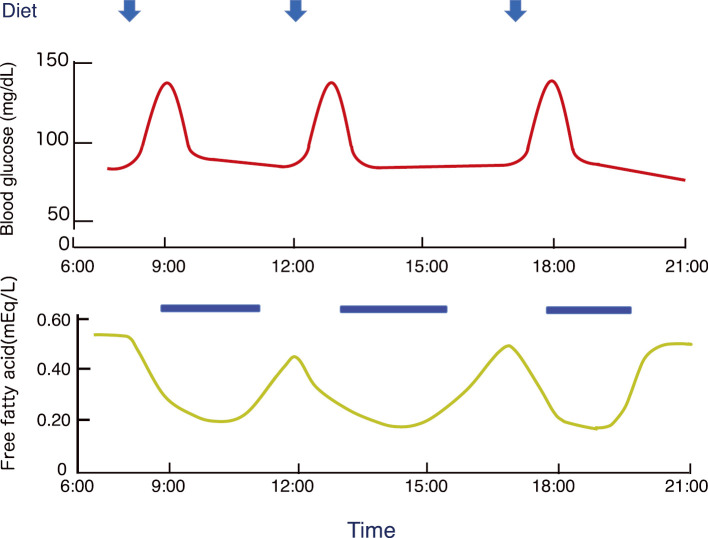
MCT supplementation is an effective treatment for CD. However, some patients exhibit irreversible growth impairment and/or brain damage, and many with CTLN2 develop irreversible liver damage soon after the clinical onset. After a meal, the level of free fatty acids, which are an essential energy source for citrin-deficient hepatocytes, decreases due to insulin secretion (Fig. 5) (5). MCT supplementation with a diet containing minimal carbohydrates (as described in the FTTDCD section) is recommended to compensate for postprandial energy deficits. The recommended minimum amount of MCT supplementation is equivalent to at least one-third (8 h) of the daily liver energy consumption (Fig. 5, Table 2). In contrast, excessive MCT supplementation has been shown to promote de novo lipogenesis, increase hepatic lipid deposition, and cause hepatic microvesicular steatosis (51). MCT supplementation should not exceed the daily calories consumed by the liver (maximum amount), and the appropriate dosage should be determined according to the patient's diet and physical activity. MCT supplementation is recommended to improve the quality of life and prevent the development of CTLN2 shortly after the diagnosis.
Figure 5.
Diurnal changes in blood glucose and plasma-free fatty acids. After a meal, blood glucose levels increase, insulin secretion is stimulated, and the concentration of free fatty acids in the plasma decreases. Blue bars indicate the energy depletion periods in citrin-deficient hepatocytes. MCT should be supplemented to provide energy to hepatocytes during the blank period (approximately 8 h per day).
Table 2.
Maximum and Minimum Recommended Dosage of MCT Supplementation.
| Life stage group | Age (year) | Moderate level of activity (kcal) | Amount of MCT equivalent to calories consumed by the liver (mL): maximum dosage | Amount of MCT equivalent to calories during post-prandial period (mL): minimum dosage |
|---|---|---|---|---|
| Child | 2-3 | 1,000-1,400 | 24-33 | 8-11 |
| Female | 4-8 | 1,400-1,600 | 33-38 | 11-13 |
| 9-13 | 1,600-2,000 | 38-47 | 13-16 | |
| 14-18 | 2,000 | 47 | 16 | |
| 19-30 | 2,000-2,200 | 47-52 | 16-18 | |
| 31-50 | 2,000 | 47 | 16 | |
| 51 and older | 1,800 | 43 | 15 | |
| Male | 4-8 | 1,400-1,600 | 33-38 | 11-13 |
| 9-13 | 1,800-2,200 | 43-52 | 15-18 | |
| 14-18 | 2,400-2,800 | 57-66 | 19-22 | |
| 19-30 | 2,400-2,600 | 57-62 | 19-21 | |
| 31-50 | 2,200-2,400 | 52-57 | 14-19 | |
| 51 and older | 2,000-2,200 | 47-52 | 17-18 |
The Recommended caloric intake for a moderate level of activity is adapted from the U.S. Department of Agriculture: Dietary Guidelines for Americans, 2005. The maximum dosage of MCT is equivalent to the daily caloric consumption by the liver, and the minimum dosage can provide energy during the period of postprandial hyperglycemia (8 hours).
The author states that he has no Conflict of Interest (COI).
Financial Support
The author would like to thank the coresearchers for contributing to the original research and Kissei Pharmaceutical, for providing MCT (Macton oil) for my initial study.
References
- 1.Kobayashi K, Sinasac DS, Iijima M, et al. The gene mutated in adult-onset type II citrullinaemia encodes a putative mitochondrial carrier protein. Nat Genet 22: 159-163, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Saheki T, Song YZ. Citrin deficiency. In: GeneReviewsⓇ [Internet]. [cited 2023 Jul 1]. Adam MP, Ardinger HH, Pagon RAet al. , Eds. University of Washington, Seattle, 2017. https://www.ncbi.nlm.nih.gov/books/NBK1181/ [Google Scholar]
- 3.Miyakoshi T, Takahashi T, Kato M, Watanabe M, Ito C. Abnormal citrulline metabolism of Inose-type hepatocerebral disease. Shinkei Kagaku (Bull Jpn Neurochem) 7: 88-91, 1968. (in Japanese). [Google Scholar]
- 4.Palmieri L, Pardo B, Lasorsa FM, et al. Citrin and aralar1 are Ca2+-stimulated aspartate/glutamate transporters in mitochondria. EMBO J 20: 5060-5069, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayasaka K. Metabolic basis and treatment of citrin deficiency. J Inherit Metab Dis 44: 110-117, 2020. [DOI] [PubMed] [Google Scholar]
- 6.Koshenov Z, Oflaz FE, Hirtl M, et al. Citrin mediated metabolic rewiring in response to altered basal subcellular Ca2+ homeostasis. Commun Biol 20: 76, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tavoulari S, Lacabanne D, Thangaratnarajah C, Kunji ERS. Pathogenic variants of the mitochondrial aspartate/glutamate carrier causing citrin deficiency. Trends Endocrinol Metab 33: 539-553, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tabata A, Sheng JS, Ushikaiet M, et al. Identification of 13 novel mutations including a retrotransposal insertion in SLC25A13 gene and frequency of 30 mutations found in patients with citrin deficiency. J Hum Genet 53: 534-545, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Kikuchi A, Arai-Ichinoi N, Sakamoto O, et al. Simple and rapid genetic testing for citrin deficiency by screening 11 prevalent mutations in SLC25A13. Mol Genet Metab 105: 553-558, 2012. [DOI] [PubMed] [Google Scholar]
- 10.Seifter S, Englard S. Energy metabolism. In: The Liver: Biology and Pathobiology. Vol 6. 5th ed. Arias I, Wolkoff A, Boyer Jet al. , Eds. Raven Press, New York, 2009: 1-42. [Google Scholar]
- 11.Hellerstein MK. De novo lipogenesis in humans: metabolic and regulatory aspects. Eur J Clin Nutr 53: S53-S65, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Chakravarthy MV, Pan Z, Zhu Y, Tordjman K, et al. “New” hepatic fat activates PPARα to maintain glucose, lipid, and cholesterol homeostasis. Cell Metab 1: 309-322, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Contreras AV, Torres N, Tovar AR. PPAR-α as a key nutritional and environmental sensor for metabolic adaptation. Adv Nutr 4: 439-445, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrera E, Amusquivar E. Lipid metabolism in the fetus and the newborn. Diabetes Metab Res Rev 16: 202-210, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Marcelino H, Veyrat-Durebex C, Summermatter S, et al. A role for adipose tissue de novo lipogenesis in glucose homeostasis during catch-up growth: a Randle cycle favoring fat storage. Diabetes 2: 362-372, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Numakura C, Tamiya G, Ueki M, et al. Growth impairment in individuals with citrin deficiency. J Inherit Metab Dis 42: 501-508, 2019. [DOI] [PubMed] [Google Scholar]
- 17.Komatsu M, Kimura T, Yazaki M, et al. Steatogenesis in adultonset type II citrullinemia is associated with down-regulation of PPARα. Biochim Biophys Acta 1852: 473-481, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayasaka K, Numakura C. Adult-onset type II citrullinemia: current insights and therapy. Appl Clin Genet 11: 163-170, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayasaka K, Numakura C, Toyota K, et al. Medium-chain triglyceride supplementation under a low-carbohydrate formula is a promising therapy for adult-onset type II citrullinemia. Mol Genet Metab Rep 1: 42-50, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayasaka K, Numakura C, Yamakawa M, et al. Medium-chain triglycerides supplement therapy with a low-carbohydrate formula can supply energy and enhance ammonia detoxification in the hepatocytes of patients with adult-onset type II citrullinemia. J Inher Metab Dis 41: 777-784, 2018. [DOI] [PubMed] [Google Scholar]
- 21.Hayasaka K, Numakura C, Toyota K, Kimura T. Treatment with lactose (galactose)-restricted and medium-chain triglyceride supplemented formula for neonatal intrahepatic cholestasis caused by citrin deficiency. JIMD Rep 2: 37-44, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saheki T, Kobayashi K. Mitochondrial aspartate glutamate carrier (citrin) deficiency as the cause of adult-onset type II citrullinemia (CTLN2) and idiopathic neonatal hepatitis (NICCD). J Hum Genet 47: 333-341, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Saheki T, Kobayashi K, Iijima M, et al. Adult-onset type II citrullinemia and idiopathic neonatal hepatitis caused by citrin deficiency: involvement of the aspartate glutamate carrier for urea synthesis and maintenance of the urea cycle. Mol Genet Metab 81: S20-S26, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Tazawa Y, Kobayashi K, Ohura T, et al. Infantile cholestatic jaundice associated with adult-onset type II citrullinemia. J Pediatr 138: 735-740, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe Y, Numakura C, Tahara T, et al. Diabetes mellitus exacerbates citrin deficiency via glucose toxicity. Diabetes Res Clin Pract 164: 108159, 2020. [DOI] [PubMed] [Google Scholar]
- 26.Sakai M, Wang T, Otoyama Y, et al. A case of adult-onset type 2 citrullinemia brought on by changes in disease-specific eating habits following resection of a large portion of the small intestine. Kanzo (Acta Hepatol Jpn) 61: 204-212, 2020. [Google Scholar]
- 27.Koda K, Akaogi M, Sekiya H, et al. A case of adult-onset type II citrullinemia triggered by entering a nursing home with a good response to medium-chain triglyceride oil therapy. Rinsho Shinkeigaku (Clin Neurol) 61: 200-203, 2021. (in Japanese). [DOI] [PubMed] [Google Scholar]
- 28.Yanai A, Moriya M, Miyamori T, et al. An adult patient with intellectual disability diagnosed as adult-onset type II citrullinemia. Nihon Shonika Gakkai Zasshi (J Jpn Pediatr Soc) 126: 515-519, 2022. (in Japanese). [Google Scholar]
- 29.Suzuki T, Matsuura K, Imura N, et al. A case of adult-onset citrullinemia developed under dietary restrictions during imprisonment. Intern Med. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yasuda T, Yamaguchi N, Kobayashi K, et al. Identification of two novel mutations in the SLC25A13 gene and detection of seven mutations in 102 patients with adult-onset type II citrullinemia. Hum Genet 107: 537-545, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Häussinger D, Schliess F. Glutamine metabolism and signaling in the liver. Front Biosci 12: 371-391, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Häberle J, Pauli S, Linnebank M, et al. Structure of the human argininosuccinate synthetase gene and an improved system for molecular diagnostics in patients with classical and mild citrullinemia. Hum Genet 110: 327-333, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Häberle J, Pauli S, Schmidt E, Schulze-Eilfing B, Berning C, Koch HG. Mild citrullinemia in Caucasians is an allelic variant of argininosuccinate synthetase deficiency (citrullinemia type 1). Mol Genet Metab 80: 302-306, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Eguchi K, Yonezawa M, Mitsui Y, Hiramatsu Y. Developmental changes of glutamate dehydrogenase activity in rat liver mitochondria and its enhancement by branched-chain amino acids. Biol Neonate 62: 83-88, 1992. [DOI] [PubMed] [Google Scholar]
- 35.Ibrahim SH, Balistreri WF. Mitochondrial hepatopathies. In: Nelson Textbook of Pediatrics. 21st ed. Kliegman RM, St Geme JW, Nathan Jet al. , Eds. Elsevier, Philadelphia, 2020: 2123-2127. [Google Scholar]
- 36.Yagi Y, Saheki T, Imamura Y, et al. The heterogeneous distribution of argininosuccinate synthetase in the liver of type II citrullinemic patients. Its specificity and possible clinical implications. Am J Clin Pathol 89: 735-741, 1998. [DOI] [PubMed] [Google Scholar]
- 37.Thompson MD, Monga SP. WNT/β-catenin signaling in liver health and disease. Hepatology 45: 1298-1305, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Yi CX, la Fleur SE, Fliers E, Kalsbeek A. The role of the autonomic nervous liver innervation in the control of energy metabolism. Biochim Biophys Acta 1802: 416-431, 2010. [DOI] [PubMed] [Google Scholar]
- 39.Naito E, Ito M, Matsuura S, et al. Type II citrullinaemia (citrin deficiency) in a neonate with hypergalactosaemia detected by mass screening. J Inherit Metab Dis 25: 71-76, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Tamakawa S, Nakamura H, Katano T, Yoshizawa M, Ohtake K, Kubota T. Hyperalimentation therapy produces a comatose state in a patient with citrullinemia. Nihon Shuchu Chiryo Igakkai Zasshi (J Jpn Soc Intensive Care Med) 1: 37-41, 1994. (in Japanese). [Google Scholar]
- 41.Yazaki M, Takei Y, Kobayashi K, Saheki T, Ikeda S. Risk of worsened encephalopathy after intravenous glycerol therapy in patients with adult onset type II citrullinemia (CTLN2). Intern Med 44: 188-195, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Moore MC, Coate KC, Winnick JJ, An Z, Cherrington AD. Regulation of hepatic glucose uptake and storage in vivo. Adv Nutr 3: 286-294, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yazaki M, Hashikura Y, Takei Y, et al. Feasibility of auxiliary partial orthotopic liver transplantation from living donors for patients with adult-onset type II citrullinemia. Liver Transpl 10: 550-554, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Steinmann B, Gitzelmann R, Van den Berghe G. Disorders of fructose metabolism. In: Metabolic and Molecular Bases of Inherited Disease. Valle D, Antonarakis ALS, Ballabio A, Beaudet AL, Mitchell GA, Eds. The Online McGraw-Hill, New York, 2019: . [Google Scholar]
- 45.Taylor SR, Ramsamooj S, Liang RJ, et al. Dietary fructose improves intestinal cell survival and nutrient absorption. Nature 597: 263-267, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nagasaka H, Okano Y, Tsukahara H, Shigematsu Y, Momoi T, Yorifuji J. Sustaining hypercitrullinemia, hypercholesterolemia and augmented oxidative stress in Japanese children with aspartate/glutamate carrier isoform 2-citrin-deficiency even during the silent period. Mol Genet Metab 97: 21-26, 2009. [DOI] [PubMed] [Google Scholar]
- 47.Hirayama S, Nagasaka H, Honda A, et al. Cholesterol metabolism is enhanced in the liver and brain of children with citrin deficiency. J Clin Endocrinol Metab 103: 2488-2497, 2018. [DOI] [PubMed] [Google Scholar]
- 48.Colgan SM, Tang D, Werstuck GH, Austin RC. Endoplasmic reticulum stress causes the activation of sterol regulatory element binding protein-2. Int J Biochem Cell Biol 39: 1843-1851, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Okano Y, Okamoto M, Yazaki M, et al. Analysis of daily energy, protein, fat, and carbohydrate intake in citrin-deficient patients: towards prevention of adult-onset type II citrullinemia. Mol Genet Metab 133: 63-70, 2021. [DOI] [PubMed] [Google Scholar]
- 50.Yazaki M. Pathogenesis and treatment of adult-onset type 2 citrullinemia (CTLN2). In: Annual Review Neurology. 2021. Suzuki N, Araki N, Ugawa Y, Kuwabara S, Shiokawa Y, Eds. Chugai-Igakusya, Tokyo, 2021: 264-270 (in Japanase). [Google Scholar]
- 51.Goetzman ES, Bharathi SS, Zhang Y, et al. Impaired mitochondrial medium chain fatty acid oxidation drives periportal microvesicular steatosis in sirtuin-5 knockout mice. Sci Rep 10: 18367, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]