Abstract
A nucleotide sequence identical with that of the recently identified murine pancreatic ribonuclease (RNAase) was isolated from a murine spleen cDNA library. Active RNAase was expressed and secreted from Escherichia coli lon-htpr- transformed with a plasmid containing the E. coli trp promoter followed by the murine RNAase gene sequence, including the original eukaryotic 26-amino-acid signal sequence. Approx. 1 mg of properly matured RNAase protein/litre was secreted into the medium of a fermentor culture after the promotor was induced by tryptophan starvation. When the signal sequence was deleted from the plasmid, intracellular RNAase activity was very low and there was no significant supernatant RNAase activity. Even higher RNAase yields were obtained with a synthetic gene for bovine pancreatic ribonuclease cloned after the signal sequence of the murine gene. About 2 mg of correctly processed RNAase A/litre was isolated from the growth medium, and a further 8-10 mg of correctly processed RNAase/litre could be isolated from the soluble fraction of the cells. Thus this eukaryotic signal sequence is both recognized by the E. coli transport and processing apparatus and gives efficient secretion, as well as export, of active, mature mammalian RNAases.
Full text
PDF


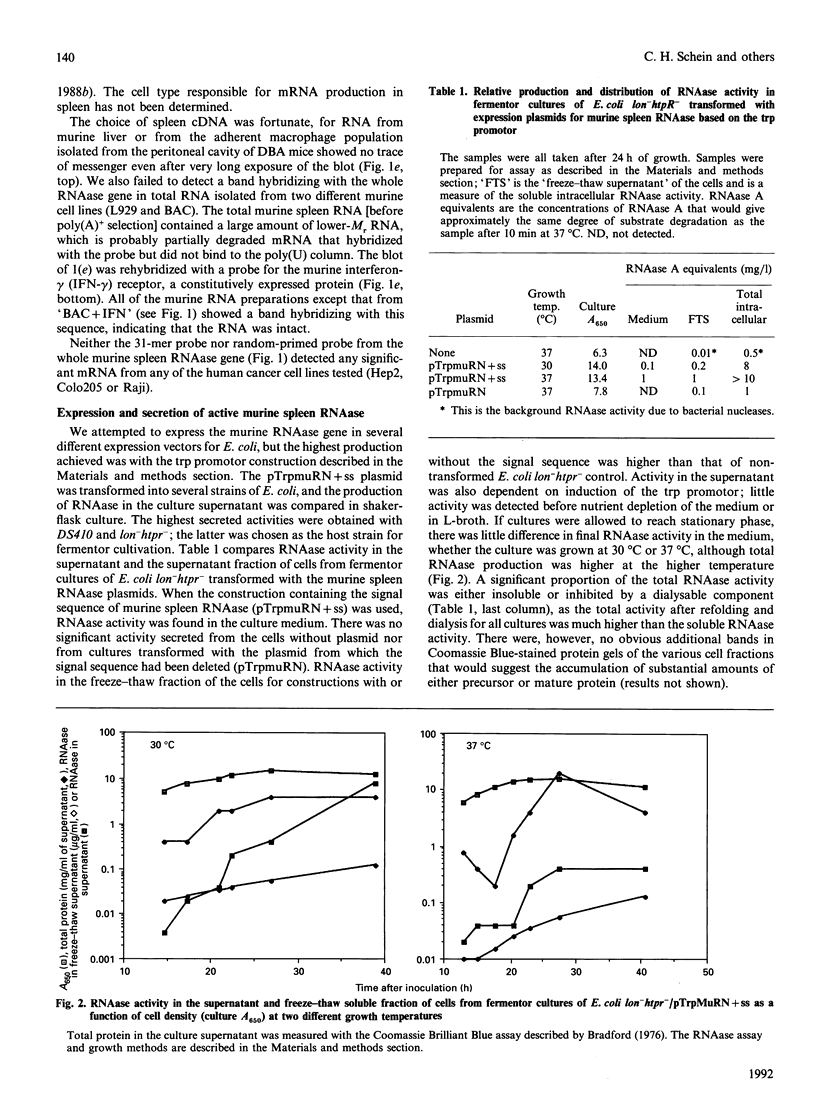
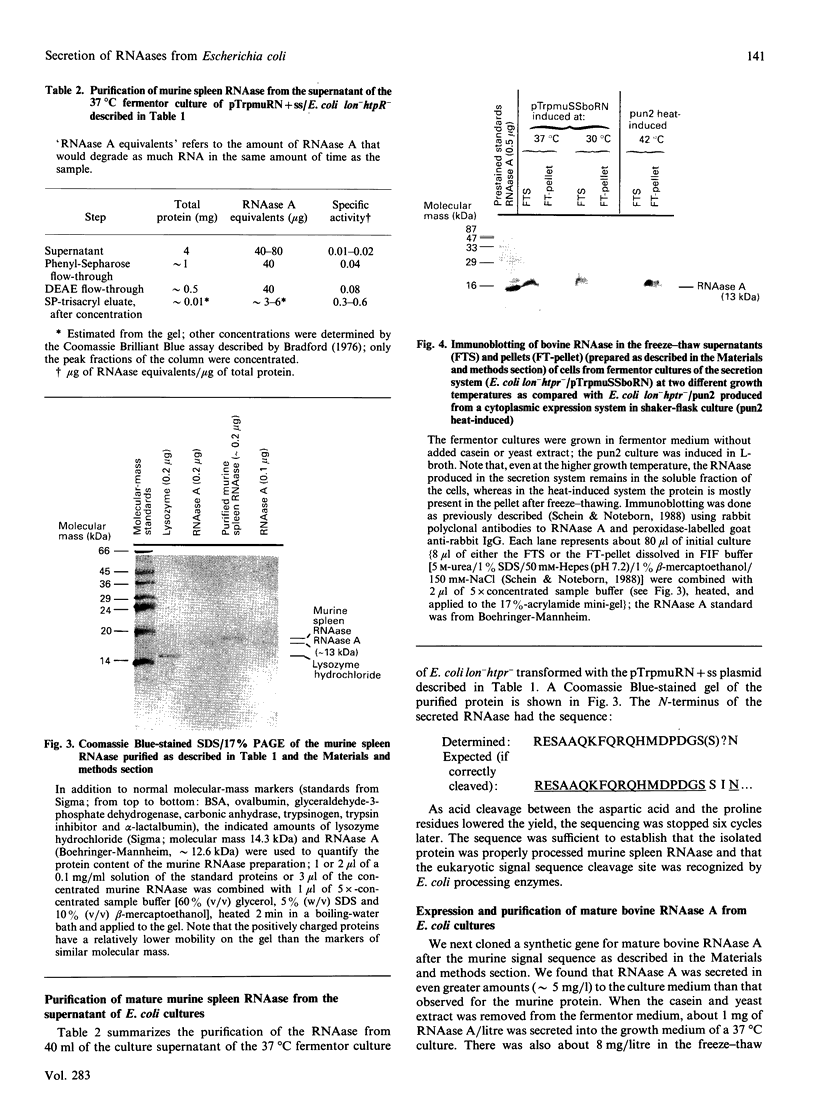
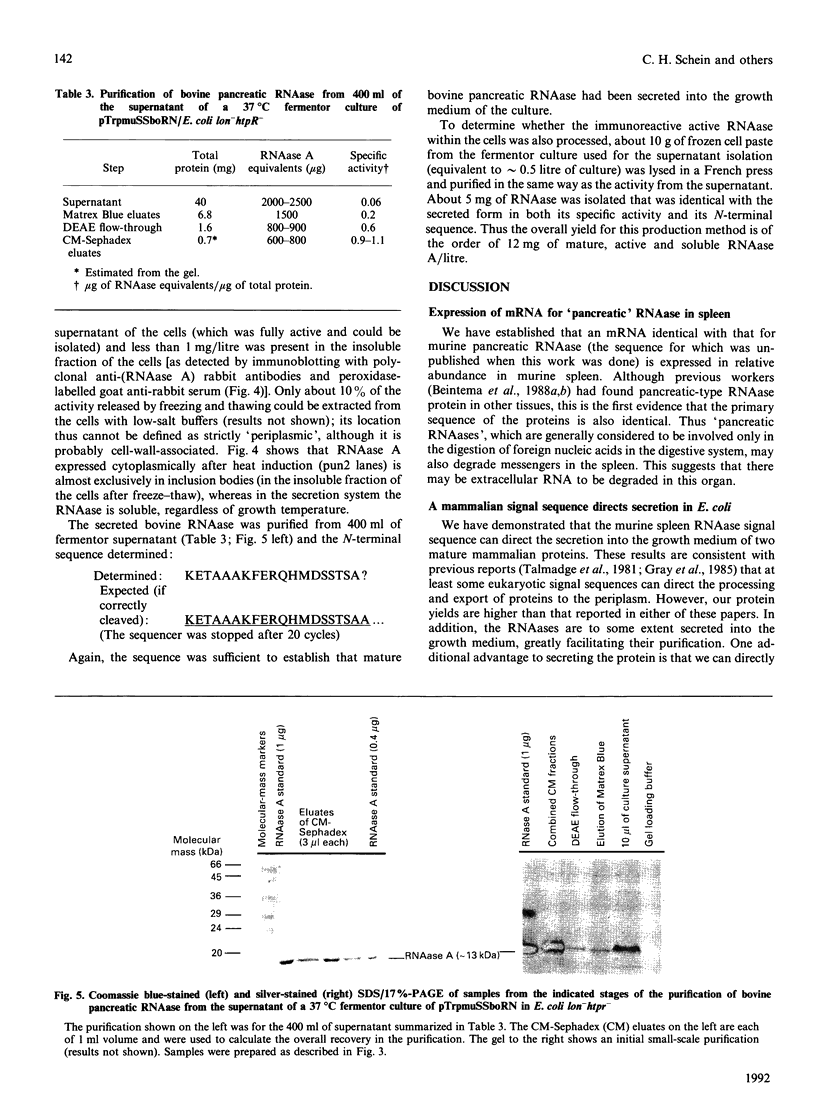


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker T. A., Grossman A. D., Gross C. A. A gene regulating the heat shock response in Escherichia coli also affects proteolysis. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6779–6783. doi: 10.1073/pnas.81.21.6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beintema J. J., Blank A., Schieven G. L., Dekker C. A., Sorrentino S., Libonati M. Differences in glycosylation pattern of human secretory ribonucleases. Biochem J. 1988 Oct 15;255(2):501–505. [PMC free article] [PubMed] [Google Scholar]
- Beintema J. J., Schüller C., Irie M., Carsana A. Molecular evolution of the ribonuclease superfamily. Prog Biophys Mol Biol. 1988;51(3):165–192. doi: 10.1016/0079-6107(88)90001-6. [DOI] [PubMed] [Google Scholar]
- Benner S. A., Allemann R. K. The return of pancreatic ribonucleases. Trends Biochem Sci. 1989 Oct;14(10):396–397. doi: 10.1016/0968-0004(89)90282-x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chang J. Y., Pai R. C., Bennett W. F., Bochner B. R. Periplasmic secretion of human growth hormone by Escherichia coli. Biochem Soc Trans. 1989 Apr;17(2):335–337. doi: 10.1042/bst0170335. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Denèfle P., Kovarik S., Ciora T., Gosselet N., Bénichou J. C., Latta M., Guinet F., Ryter A., Mayaux J. F. Heterologous protein export in Escherichia coli: influence of bacterial signal peptides on the export of human interleukin 1 beta. Gene. 1989 Dec 28;85(2):499–510. doi: 10.1016/0378-1119(89)90444-7. [DOI] [PubMed] [Google Scholar]
- Deutscher M. P. The metabolic role of RNases. Trends Biochem Sci. 1988 Apr;13(4):136–139. doi: 10.1016/0968-0004(88)90070-9. [DOI] [PubMed] [Google Scholar]
- Gierasch L. M. Signal sequences. Biochemistry. 1989 Feb 7;28(3):923–930. doi: 10.1021/bi00429a001. [DOI] [PubMed] [Google Scholar]
- Goff S. A., Casson L. P., Goldberg A. L. Heat shock regulatory gene htpR influences rates of protein degradation and expression of the lon gene in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6647–6651. doi: 10.1073/pnas.81.21.6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. L., Baldridge J. S., McKeown K. S., Heyneker H. L., Chang C. N. Periplasmic production of correctly processed human growth hormone in Escherichia coli: natural and bacterial signal sequences are interchangeable. Gene. 1985;39(2-3):247–254. doi: 10.1016/0378-1119(85)90319-1. [DOI] [PubMed] [Google Scholar]
- Hartley R. W. Barnase and barstar. Expression of its cloned inhibitor permits expression of a cloned ribonuclease. J Mol Biol. 1988 Aug 20;202(4):913–915. doi: 10.1016/0022-2836(88)90568-2. [DOI] [PubMed] [Google Scholar]
- Hemmi S., Peghini P., Metzler M., Merlin G., Dembic Z., Aguet M. Cloning of murine interferon gamma receptor cDNA: expression in human cells mediates high-affinity binding but is not sufficient to confer sensitivity to murine interferon gamma. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9901–9905. doi: 10.1073/pnas.86.24.9901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins D. G., Sharp P. M. CLUSTAL: a package for performing multiple sequence alignment on a microcomputer. Gene. 1988 Dec 15;73(1):237–244. doi: 10.1016/0378-1119(88)90330-7. [DOI] [PubMed] [Google Scholar]
- Holland I. B., Kenny B., Steipe B., Plückthun A. Secretion of heterologous proteins in Escherichia coli. Methods Enzymol. 1990;182:132–143. doi: 10.1016/0076-6879(90)82013-r. [DOI] [PubMed] [Google Scholar]
- MacDonald R. J., Stary S. J., Swift G. H. Rat pancreatic ribonuclease messenger RNA. The nucleotide sequence of the entire mRNA and the derived amino acid sequence of the pre-enzyme. J Biol Chem. 1982 Dec 25;257(24):14582–14585. [PubMed] [Google Scholar]
- Nambiar K. P., Stackhouse J., Presnell S. R., Benner S. A. Expression of bovine pancreatic ribonuclease A in Escherichia coli. Eur J Biochem. 1987 Feb 16;163(1):67–71. doi: 10.1111/j.1432-1033.1987.tb10737.x. [DOI] [PubMed] [Google Scholar]
- Perlman D., Halvorson H. O. A putative signal peptidase recognition site and sequence in eukaryotic and prokaryotic signal peptides. J Mol Biol. 1983 Jun 25;167(2):391–409. doi: 10.1016/s0022-2836(83)80341-6. [DOI] [PubMed] [Google Scholar]
- Poritz M. A., Bernstein H. D., Strub K., Zopf D., Wilhelm H., Walter P. An E. coli ribonucleoprotein containing 4.5S RNA resembles mammalian signal recognition particle. Science. 1990 Nov 23;250(4984):1111–1117. doi: 10.1126/science.1701272. [DOI] [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J. Unity in function in the absence of consensus in sequence: role of leader peptides in export. Science. 1989 Mar 3;243(4895):1156–1159. doi: 10.1126/science.2646712. [DOI] [PubMed] [Google Scholar]
- Rink H., Liersch M., Sieber P., Meyer F. A large fragment approach to DNA synthesis: total synthesis of a gene for the protease inhibitor eglin c from the leech Hirudo medicinalis and its expression in E. coli. Nucleic Acids Res. 1984 Aug 24;12(16):6369–6387. doi: 10.1093/nar/12.16.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozen K. J., Fenwick R. G., Jr, Curtiss R., 3rd Synthesis of ribonucleic acid and protein in plasmid-containing minicells of Escherichia coli K-12. J Bacteriol. 1971 Jul;107(1):21–33. doi: 10.1128/jb.107.1.21-33.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarvas M. Protein secretion in bacilli. Curr Top Microbiol Immunol. 1986;125:103–125. doi: 10.1007/978-3-642-71251-7_8. [DOI] [PubMed] [Google Scholar]
- Schein C. H., Haugg M., Benner S. A. Interferon-gamma activates the cleavage of double-stranded RNA by bovine seminal ribonuclease. FEBS Lett. 1990 Sep 17;270(1-2):229–232. doi: 10.1016/0014-5793(90)81275-s. [DOI] [PubMed] [Google Scholar]
- Schwarzbaum S., Diamond B. Generation of functional I-Ed variants from an antigen-presenting macrophage cell line. J Immunol. 1985 Jul;135(1):141–146. [PubMed] [Google Scholar]
- Schüller C., Nijssen H. M., Kok R., Beintema J. J. Evolution of nucleic acids coding for ribonucleases: the mRNA sequence of mouse pancreatic ribonuclease. Mol Biol Evol. 1990 Jan;7(1):29–44. doi: 10.1093/oxfordjournals.molbev.a040585. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Harper J. W., Fox E. A., Jansen H. W., Hein F., Uhlmann E. Expression of Met-(-1) angiogenin in Escherichia coli: conversion to the authentic less than Glu-1 protein. Anal Biochem. 1988 Dec;175(2):450–461. doi: 10.1016/0003-2697(88)90569-6. [DOI] [PubMed] [Google Scholar]
- Stader J. A., Silhavy T. J. Engineering Escherichia coli to secrete heterologous gene products. Methods Enzymol. 1990;185:166–187. doi: 10.1016/0076-6879(90)85017-i. [DOI] [PubMed] [Google Scholar]
- Summers R. G., Harris C. R., Knowles J. R. A conservative amino acid substitution, arginine for lysine, abolishes export of a hybrid protein in Escherichia coli. Implications for the mechanism of protein secretion. J Biol Chem. 1989 Nov 25;264(33):20082–20088. [PubMed] [Google Scholar]
- Talmadge K., Brosius J., Gilbert W. An 'internal' signal sequence directs secretion and processing or proinsulin in bacteria. Nature. 1981 Nov 12;294(5837):176–178. doi: 10.1038/294176a0. [DOI] [PubMed] [Google Scholar]
- Trautwein K., Holliger P., Stackhouse J., Benner S. A. Site-directed mutagenesis of bovine pancreatic ribonuclease: lysine-41 and aspartate-121. FEBS Lett. 1991 Apr 9;281(1-2):275–277. doi: 10.1016/0014-5793(91)80410-5. [DOI] [PubMed] [Google Scholar]
- Uhlén M., Abrahmsén L. Secretion of recombinant proteins into the culture medium by Escherichia coli and Staphylococcus aureus. Biochem Soc Trans. 1989 Apr;17(2):340–341. doi: 10.1042/bst0170340. [DOI] [PubMed] [Google Scholar]
- Vasantha N., Filpula D. Expression of bovine pancreatic ribonuclease A coded by a synthetic gene in Bacillus subtilis. Gene. 1989 Mar 15;76(1):53–60. doi: 10.1016/0378-1119(89)90007-3. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Net N-C charge imbalance may be important for signal sequence function in bacteria. J Mol Biol. 1986 Nov 20;192(2):287–290. doi: 10.1016/0022-2836(86)90365-7. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]