Abstract
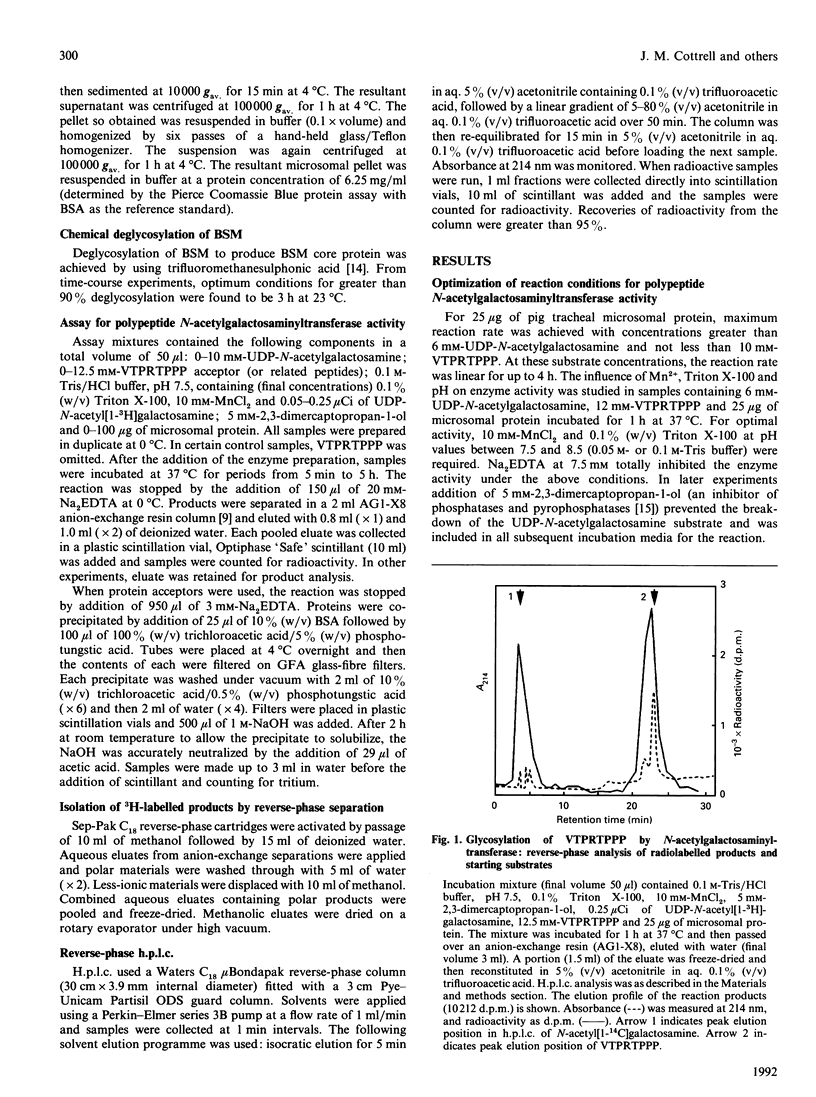
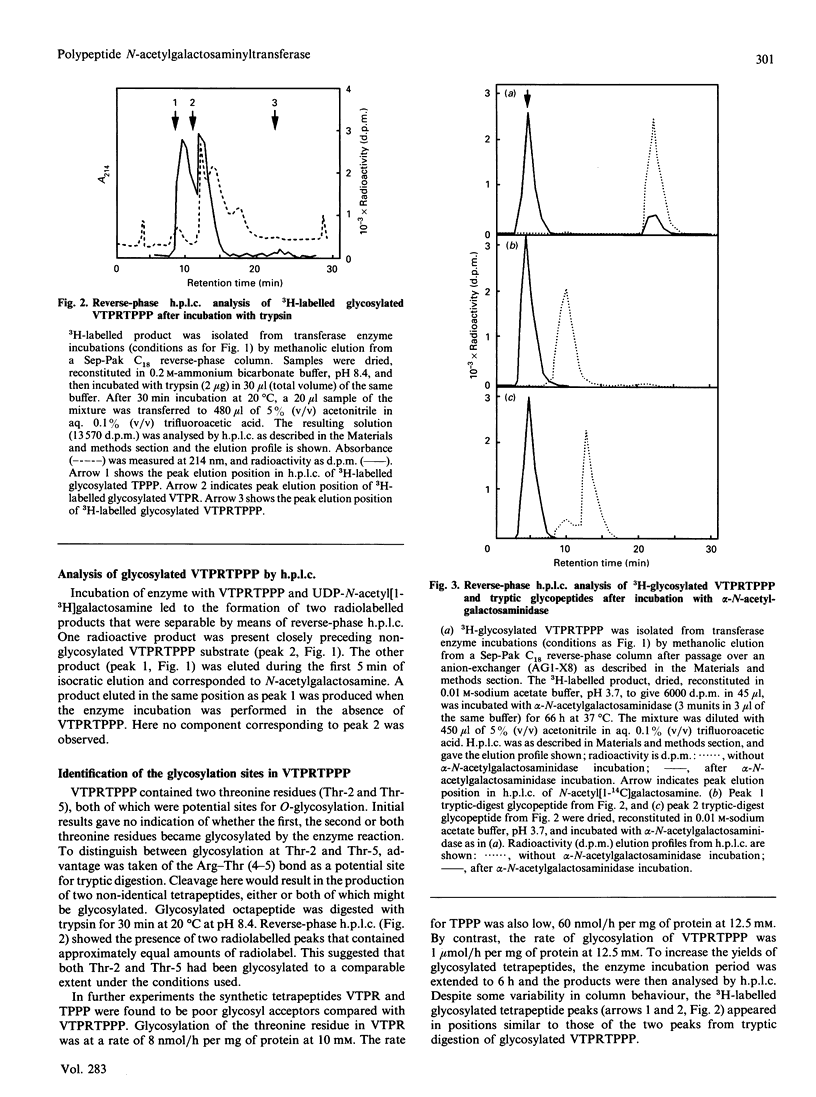
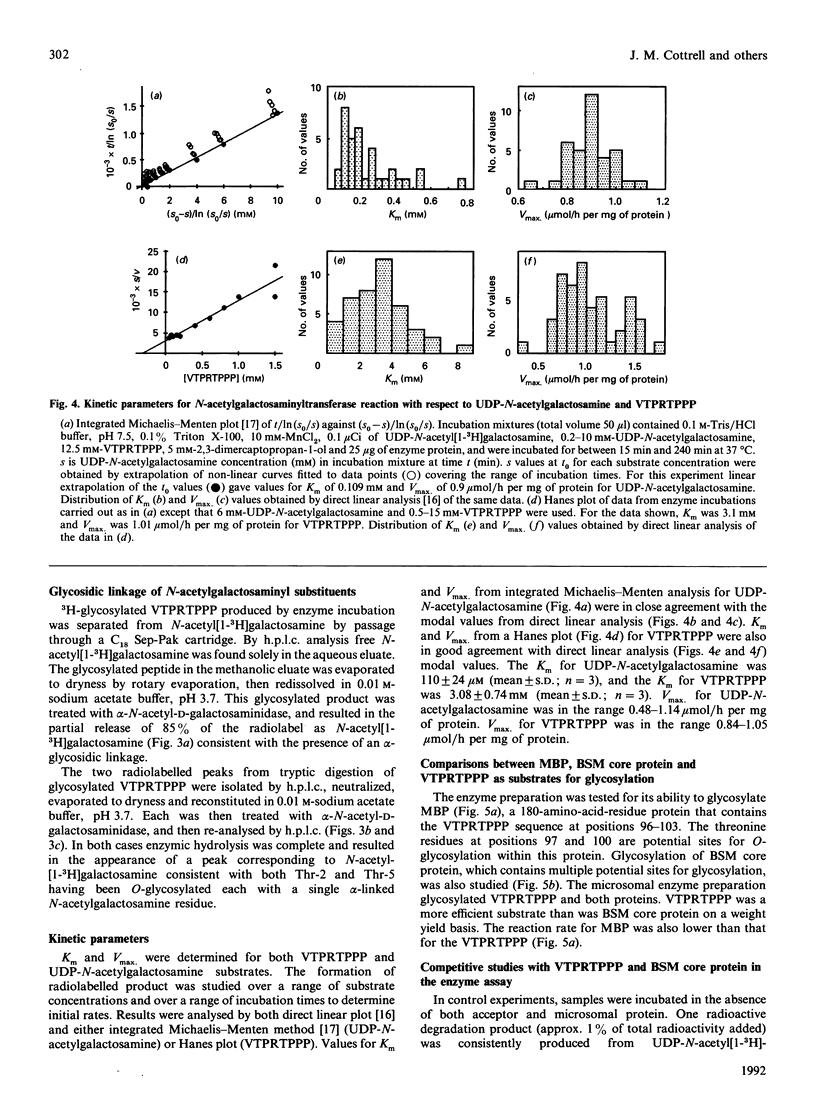
Pig tracheal epithelium, a site of extensive mucin biosynthesis, contained polypeptide N-acetylgalactosaminyltransferase activity directed towards L-threonine residues. The enzyme preparation was broadly similar in properties to preparations from other tissues, e.g. pig and bovine submaxillary glands, bovine colostrum, BW5147 mouse lymphoma and baby-hamster kidney cells. Enzyme was membrane-bound and was released from microsomal preparations by extraction with Triton X-100. Extracted enzyme had a pH optimum of 7.5, had a requirement for Mn2+ (10 mM) and was inhibited by Na2EDTA. The Km for UDP-N-acetylgalactosamine was 110 microM and that for an octapeptide acceptor (VTPRTPPP) was 3.0 mM at 37 degrees C. Using a range of synthetic peptides of known structure related to TPPP it was established that L-threonine residues were specifically O-glycosylated probably in the alpha-configuration. Synthetic peptides containing the TPPP sequence required a peptide length of five or more for significant acceptor activity. In VTPRTPPP the two threonine residues were similarly glycosylated, as revealed by tryptic cleavage of the glycosylated product and separation of the 3H-labelled fragments. The enzyme preparation also specifically catalysed the transfer of N-acetylgalactosaminyl residues from UDP-N-acetyl[1-3H]galactosamine to bovine submaxillary mucin core protein and to myelin basic protein.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker A. P., Sawyer J. L. Glycosyltransferases in human respiratory tissue. Alterations in subjects with hypersecretion of mucus. Biochem Med. 1975 Sep;14(1):42–50. doi: 10.1016/0006-2944(75)90018-6. [DOI] [PubMed] [Google Scholar]
- Bhargava A. K., Woitach J. T., Davidson E. A., Bhavanandan V. P. Cloning and cDNA sequence of a bovine submaxillary gland mucin-like protein containing two distinct domains. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6798–6802. doi: 10.1073/pnas.87.17.6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briand J. P., Andrews S. P., Jr, Cahill E., Conway N. A., Young J. D. Investigation of the requirements for O-glycosylation by bovine submaxillary gland UDP-N-acetylgalactosamine:polypeptide N-acetylgalactosamine transferase using synthetic peptide substrates. J Biol Chem. 1981 Dec 10;256(23):12205–12207. [PubMed] [Google Scholar]
- Carraway K. L., Hull S. R. O-glycosylation pathway for mucin-type glycoproteins. Bioessays. 1989 Apr;10(4):117–121. doi: 10.1002/bies.950100406. [DOI] [PubMed] [Google Scholar]
- Edge A. S., Faltynek C. R., Hof L., Reichert L. E., Jr, Weber P. Deglycosylation of glycoproteins by trifluoromethanesulfonic acid. Anal Biochem. 1981 Nov 15;118(1):131–137. doi: 10.1016/0003-2697(81)90168-8. [DOI] [PubMed] [Google Scholar]
- Eisenthal R., Cornish-Bowden A. The direct linear plot. A new graphical procedure for estimating enzyme kinetic parameters. Biochem J. 1974 Jun;139(3):715–720. doi: 10.1042/bj1390715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhammer A., Kornfeld S. Purification and characterization of UDP-N-acetylgalactosamine: polypeptide N-acetylgalactosaminyltransferase from bovine colostrum and murine lymphoma BW5147 cells. J Biol Chem. 1986 Apr 25;261(12):5249–5255. [PubMed] [Google Scholar]
- Elhammer A., Kornfeld S. Two enzymes involved in the synthesis of O-linked oligosaccharides are localized on membranes of different densities in mouse lymphoma BW5147 cells. J Cell Biol. 1984 Jul;99(1 Pt 1):327–331. doi: 10.1083/jcb.99.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faltynek C. R., Silbert J. E., Hof L. Inhibition of the action of pyrophosphatase and phosphatase on sugar nucleotides. J Biol Chem. 1981 Jul 25;256(14):7139–7141. [PubMed] [Google Scholar]
- Fiers W., Contreras R., Haegemann G., Rogiers R., Van de Voorde A., Van Heuverswyn H., Van Herreweghe J., Volckaert G., Ysebaert M. Complete nucleotide sequence of SV40 DNA. Nature. 1978 May 11;273(5658):113–120. doi: 10.1038/273113a0. [DOI] [PubMed] [Google Scholar]
- Hagopian A., Bosmann H. B., Eylar E. H. Glycoprotein biosynthesis: the localization of polypeptidyl: N-acetylgalactosaminyl, collagen: glucosyl, and glycoprotein:galactosyl transferases in HeLa cell membrane fractions. Arch Biochem Biophys. 1968 Nov;128(2):387–396. doi: 10.1016/0003-9861(68)90045-3. [DOI] [PubMed] [Google Scholar]
- Hagopian A., Eylar E. H. Glycoprotein biosynthesis: studies on the receptor specificity of the polypeptidyl: N-acetylgalactosaminyl transferase from bovine submaxillary glands. Arch Biochem Biophys. 1968 Nov;128(2):422–433. doi: 10.1016/0003-9861(68)90048-9. [DOI] [PubMed] [Google Scholar]
- Hanover J. A., Elting J., Mintz G. R., Lennarz W. J. Temporal aspects of the N- and O-glycosylation of human chorionic gonadotropin. J Biol Chem. 1982 Sep 10;257(17):10172–10177. [PubMed] [Google Scholar]
- Hill H. D., Jr, Schwyzer M., Steinman H. M., Hill R. L. Ovine submaxillary mucin. Primary structure and peptide substrates of UDP-N-acetylgalactosamine:mucin transferase. J Biol Chem. 1977 Jun 10;252(11):3799–3804. [PubMed] [Google Scholar]
- Hughes R. C., Bradbury A. F., Smyth D. G. Substrate recognition by UDP-N-acetyl-alpha-D-galactosamine: polypeptide n-acetyl-alpha-D-galactosaminyltransferase. Effects of chain length and disulphide bonding of synthetic peptide substrates. Carbohydr Res. 1988 Jul 15;178:259–269. doi: 10.1016/0008-6215(88)80117-4. [DOI] [PubMed] [Google Scholar]
- Kim Y. S., Perdomo J., Nordberg J. Glycoprortein biosynthesis in small intestinal mucosa. I. A study of glycosyltransferases in microsomal subfractions. J Biol Chem. 1971 Sep 10;246(17):5466–5476. [PubMed] [Google Scholar]
- Ko G. K., Raghupathy E. Glycoprotein biosynthesis in the developing rat brain. II. Microsomal galactosaminyltransferase utilizing endogenous and exogenous protein acceptors. Biochim Biophys Acta. 1972 Mar 30;264(1):129–143. doi: 10.1016/0304-4165(72)90124-9. [DOI] [PubMed] [Google Scholar]
- Ko G. K., Raghupathy E. UDP-N-acetylgalactosamine:protein N-acetylgalactosaminyl transferase activity of human sera. Biochem Biophys Res Commun. 1972 Feb 25;46(4):1704–1712. doi: 10.1016/0006-291x(72)90806-6. [DOI] [PubMed] [Google Scholar]
- Stojanovic D., Vischer P., Hughes R. C. Glycosyl transferases of baby hamster kidney cells and ricin-resistant mutants. O-glycan biosynthesis. Eur J Biochem. 1984 Feb 1;138(3):551–562. doi: 10.1111/j.1432-1033.1984.tb07950.x. [DOI] [PubMed] [Google Scholar]
- Sugiura M., Kawasaki T., Yamashina I. Purification and characterization of UDP-GalNAc:polypeptide N-acetylgalactosamine transferase from an ascites hepatoma, AH 66. J Biol Chem. 1982 Aug 25;257(16):9501–9507. [PubMed] [Google Scholar]
- Wilson I. B., Gavel Y., von Heijne G. Amino acid distributions around O-linked glycosylation sites. Biochem J. 1991 Apr 15;275(Pt 2):529–534. doi: 10.1042/bj2750529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfenstein-Todel C., Frangione B., Prelli F., Franklin E. C. The amino acid sequence of "heavy chain disease" protein ZUC. Structure of the Fc fragment of immunoglobulin G3. Biochem Biophys Res Commun. 1976 Aug 23;71(4):907–914. doi: 10.1016/0006-291x(76)90741-5. [DOI] [PubMed] [Google Scholar]
- Young J. D., Tsuchiya D., Sandlin D. E., Holroyde M. J. Enzymic O-glycosylation of synthetic peptides from sequences in basic myelin protein. Biochemistry. 1979 Oct 2;18(20):4444–4448. doi: 10.1021/bi00587a026. [DOI] [PubMed] [Google Scholar]


