Abstract
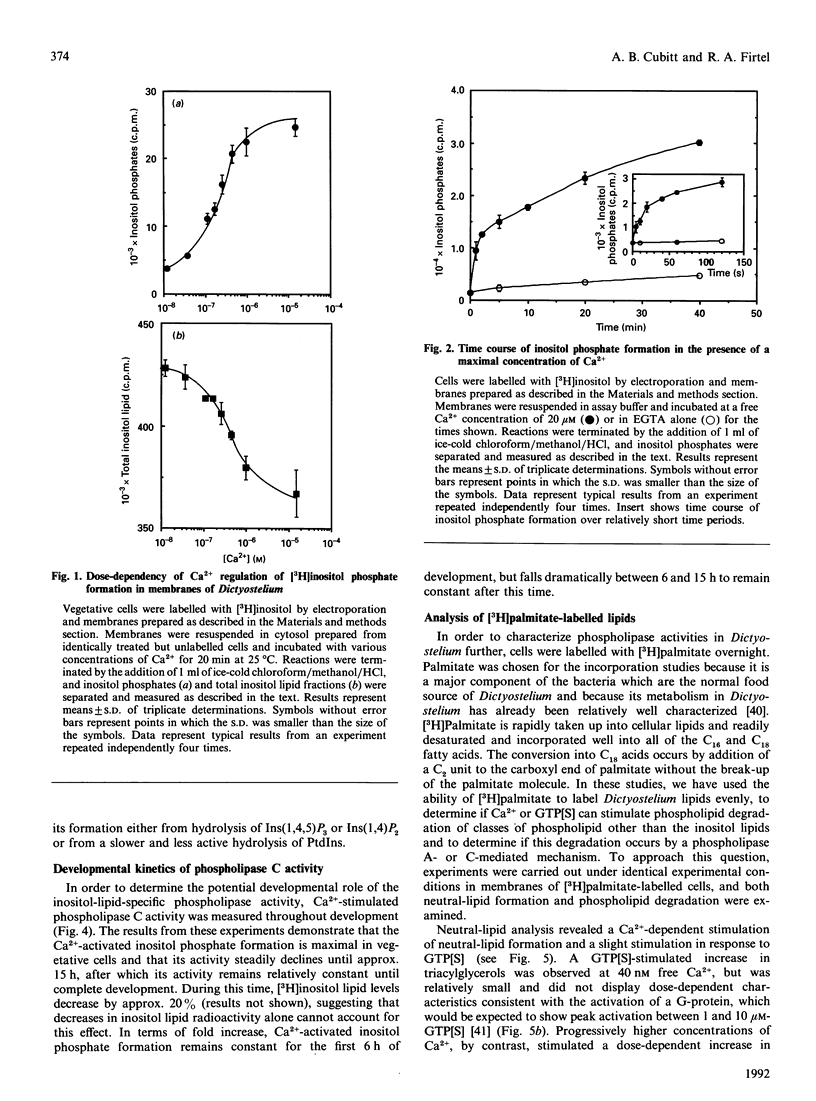
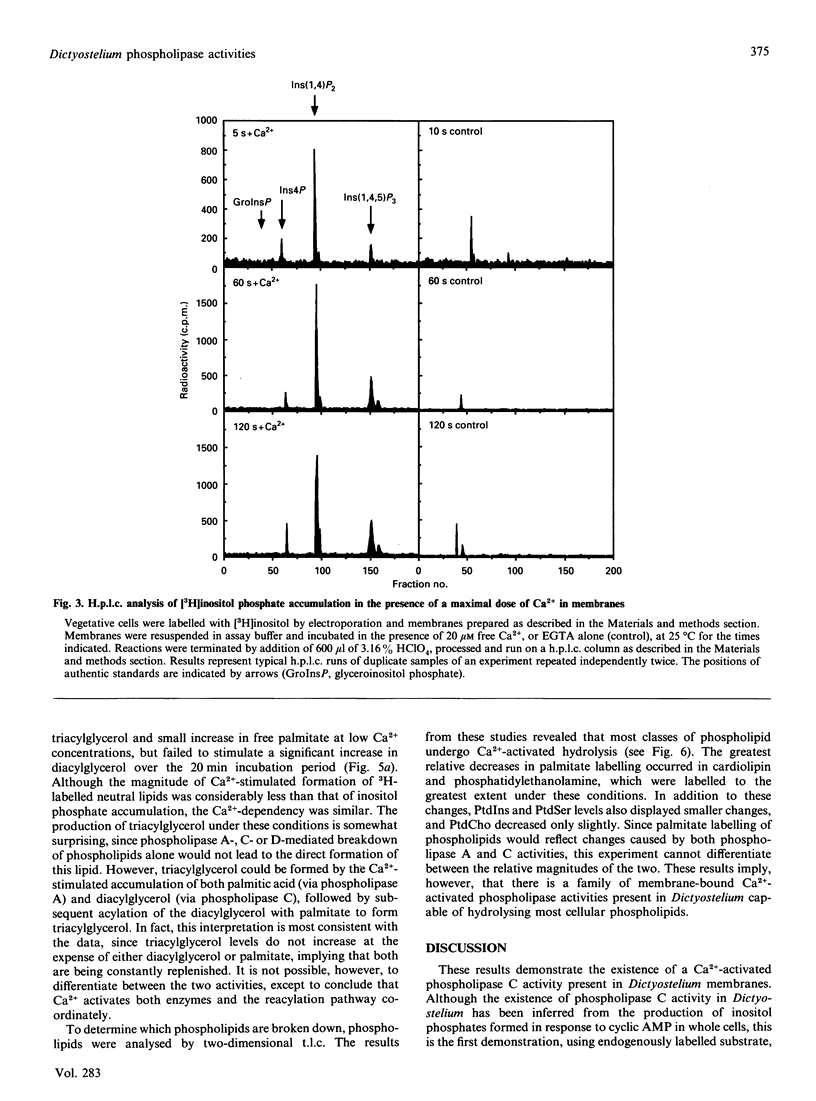
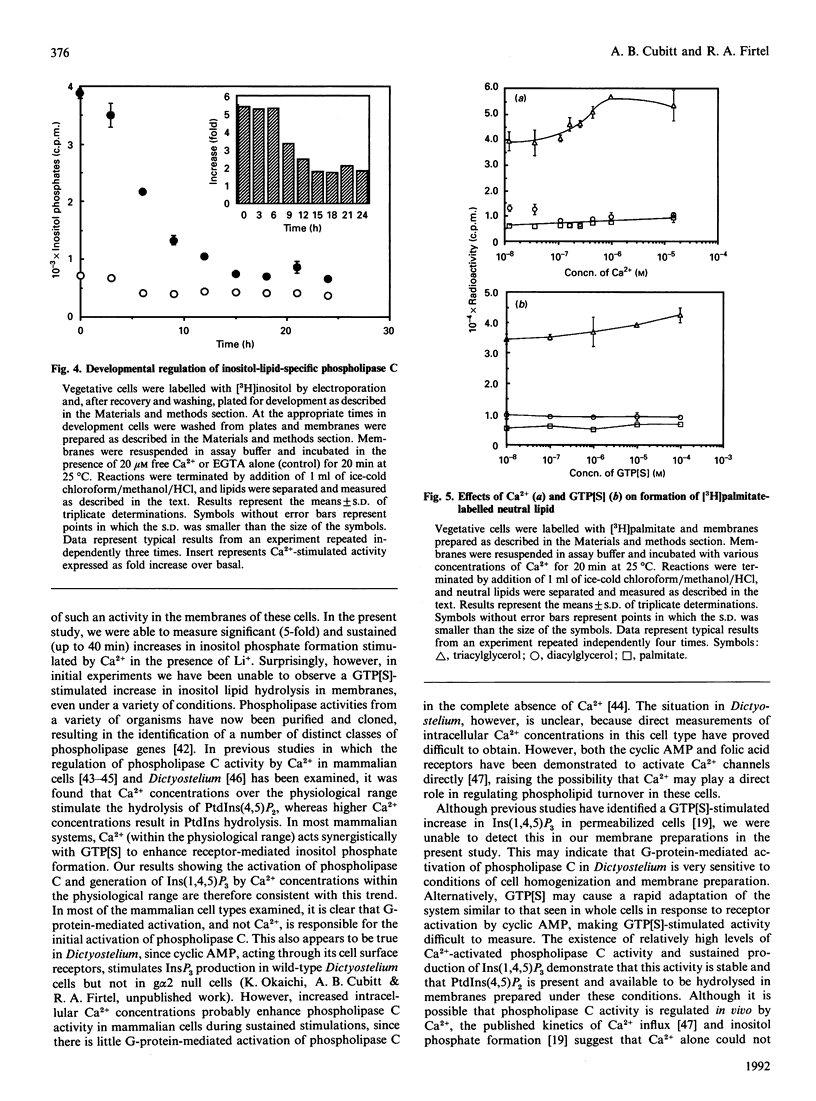
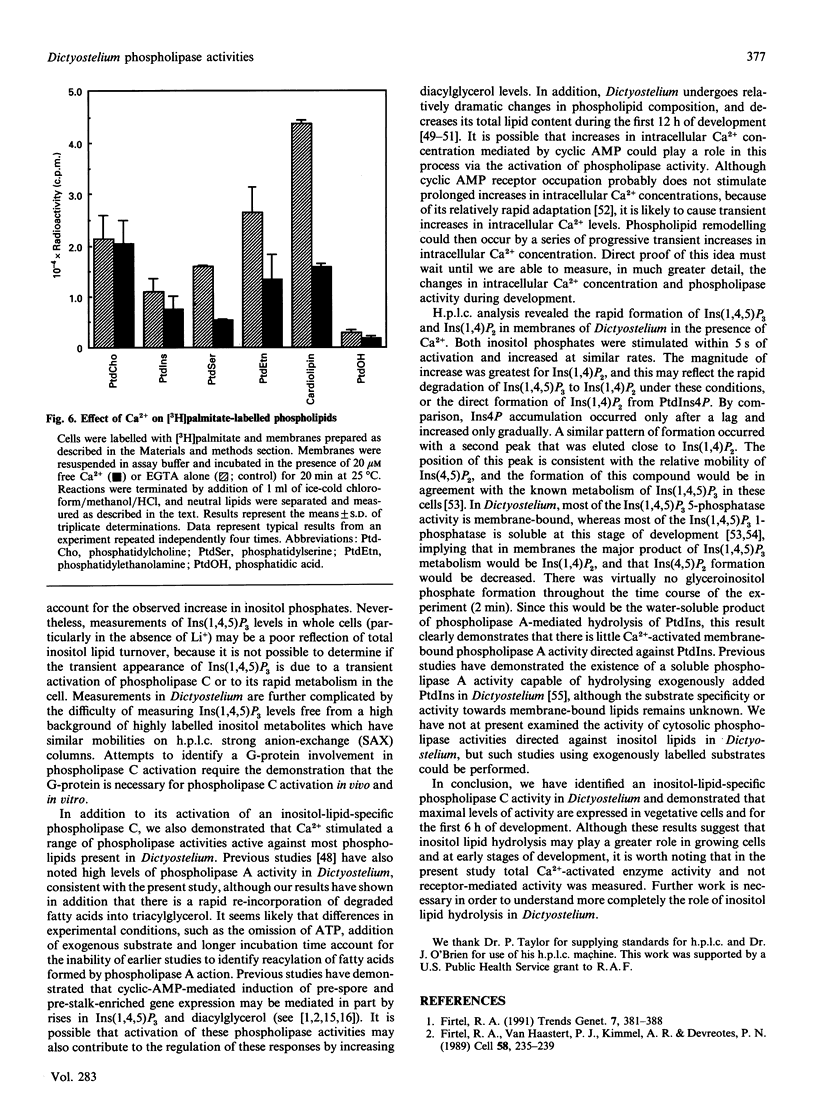
We have identified a Ca(2+)-dependent polyphosphoinositide-specific phospholipase C activity in Dictyostelium discoideum. Addition of Ca2+ (20 microM) results in the rapid formation of Ins(1,4,5)P3 within 5 s and leads to sustained inositol phosphate production for up to 40 min in membranes prepared from [3H]inositol-labelled cells. The phospholipase C activity is primarily membrane-bound under the conditions used to lyse the cells. In addition to this activity we also identified a family of Ca(2+)-regulated phospholipase activities active on a range of phospholipid substrates, using [3H]palmitate labelling. Inositol-specific phospholipase C activity is highest in vegetatively growing cells and in starved cells during the first 6 h in development, during which time Ca2+ elicited a 5-fold stimulation of inositol phosphate formation. After this time, total activity decreased progressively until 15 h, after which the activity remained constant up until 24 h. During this period, Ca2+ was able to stimulate a 2-fold increase in inositol phosphates.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe T., Maeda Y., Iijima T. Transient increase of the intracellular Ca2+ concentration during chemotactic signal transduction in Dictyostelium discoideum cells. Differentiation. 1988 Dec;39(2):90–96. doi: 10.1111/j.1432-0436.1988.tb00085.x. [DOI] [PubMed] [Google Scholar]
- Bominaar A. A., Van Dijken P., Draijer R., Van Haastert P. J. Developmental regulation of the inositol 1,4,5-trisphosphate phosphatases in Dictyostelium discoideum. Differentiation. 1991 Feb;46(1):1–5. doi: 10.1111/j.1432-0436.1991.tb00859.x. [DOI] [PubMed] [Google Scholar]
- Bominaar A. A., van der Kaay J., van Haastert P. J. Dynamics and function of the inositolcycle in Dictyostelium discoideum. Dev Genet. 1991;12(1-2):19–24. doi: 10.1002/dvg.1020120106. [DOI] [PubMed] [Google Scholar]
- Boyer J. L., Waldo G. L., Evans T., Northup J. K., Downes C. P., Harden T. K. Modification of AlF-4- and receptor-stimulated phospholipase C activity by G-protein beta gamma subunits. J Biol Chem. 1989 Aug 15;264(23):13917–13922. [PubMed] [Google Scholar]
- Carter H. R., Wallace M. A., Fain J. N. Purification and characterization of PLC-beta m, a muscarinic cholinergic regulated phospholipase C from rabbit brain membrane. Biochim Biophys Acta. 1990 Aug 13;1054(1):119–128. doi: 10.1016/0167-4889(90)90213-w. [DOI] [PubMed] [Google Scholar]
- DAVIDOFF F., KORN E. D. FATTY ACID AND PHOSPHOLIPID COMPOSITION OF THE CELLULAR SLIME MOLD, DICTYOSTELIUM DISCOIDEUM. THE OCCURRENCE OF PREVIOUSLY UNDESCRIBED FATTY ACIDS. J Biol Chem. 1963 Oct;238:3199–3209. [PubMed] [Google Scholar]
- DAVIDOFF F., KORN E. D. THE BIOSYNTHESIS OF FATTY ACIDS IN THE CELLULAR SLIME MOLD, DICTYOSTELIUM DISCOIDEUM. J Biol Chem. 1963 Oct;238:3210–3215. [PubMed] [Google Scholar]
- Diaz-Laviada I., Larrodera P., Nieto J. L., Cornet M. E., Diaz-Meco M. T., Sanchez M. J., Guddal P. H., Johansen T., Haro A., Moscat J. Mechanism of inhibition of adenylate cyclase by phospholipase C-catalyzed hydrolysis of phosphatidylcholine. Involvement of a pertussis toxin-sensitive G protein and protein kinase C. J Biol Chem. 1991 Jan 15;266(2):1170–1176. [PubMed] [Google Scholar]
- Ellingson J. S. Changes in the phospholipid composition in the differentiating cellular slime mold, Dictyostelium discoideum. Biochim Biophys Acta. 1974 Jan 23;337(1):60–67. doi: 10.1016/0005-2760(74)90040-x. [DOI] [PubMed] [Google Scholar]
- Europe-Finner G. N., Ludérus M. E., Small N. V., Van Driel R., Reymond C. D., Firtel R. A., Newell P. C. Mutant ras gene induces elevated levels of inositol tris- and hexakisphosphates in Dictyostelium. J Cell Sci. 1988 Jan;89(Pt 1):13–20. doi: 10.1242/jcs.89.1.13. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Signaling through phosphatidylcholine breakdown. J Biol Chem. 1990 Jan 5;265(1):1–4. [PubMed] [Google Scholar]
- Ferber E., Munder P. G., Fischer H., Gerisch G. High phospholipase activities in amoebae of Dictyostelium discoideum. Eur J Biochem. 1970 Jun;14(2):253–257. doi: 10.1111/j.1432-1033.1970.tb00284.x. [DOI] [PubMed] [Google Scholar]
- Firtel R. A. Signal transduction pathways controlling multicellular development in Dictyostelium. Trends Genet. 1991 Nov-Dec;7(11-12):381–388. doi: 10.1016/0168-9525(91)90260-w. [DOI] [PubMed] [Google Scholar]
- Firtel R. A., van Haastert P. J., Kimmel A. R., Devreotes P. N. G protein linked signal transduction pathways in development: dictyostelium as an experimental system. Cell. 1989 Jul 28;58(2):235–239. doi: 10.1016/0092-8674(89)90837-4. [DOI] [PubMed] [Google Scholar]
- Fournié J. J., Mullins R. J., Basten A. Isolation and structural characteristics of a monoclonal antibody-defined cross-reactive phospholipid antigen from Mycobacterium tuberculosis and Mycobacterium leprae. J Biol Chem. 1991 Jan 15;266(2):1211–1219. [PubMed] [Google Scholar]
- Ginsburg G., Kimmel A. R. Inositol trisphosphate and diacylglycerol can differentially modulate gene expression in Dictyostelium. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9332–9336. doi: 10.1073/pnas.86.23.9332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberstroh L., Firtel R. A. A spatial gradient of expression of a cAMP-regulated prespore cell-type-specific gene in Dictyostelium. Genes Dev. 1990 Apr;4(4):596–612. doi: 10.1101/gad.4.4.596. [DOI] [PubMed] [Google Scholar]
- Hadwiger J. A., Wilkie T. M., Strathmann M., Firtel R. A. Identification of Dictyostelium G alpha genes expressed during multicellular development. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8213–8217. doi: 10.1073/pnas.88.18.8213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hase A. Changes in phospholipid composition during the development of Dictyostelium discoideum. Arch Biochem Biophys. 1982 Nov;219(1):21–29. doi: 10.1016/0003-9861(82)90129-1. [DOI] [PubMed] [Google Scholar]
- Howard P. K., Ahern K. G., Firtel R. A. Establishment of a transient expression system for Dictyostelium discoideum. Nucleic Acids Res. 1988 Mar 25;16(6):2613–2623. doi: 10.1093/nar/16.6.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai A., Gershengorn M. C. Measurement of lipid turnover in response to thyrotropin-releasing hormone. Methods Enzymol. 1987;141:100–101. doi: 10.1016/0076-6879(87)41059-8. [DOI] [PubMed] [Google Scholar]
- Imai A., Gershengorn M. C. Phosphatidylinositol 4,5-bisphosphate turnover is transient while phosphatidylinositol turnover is persistent in thyrotropin-releasing hormone-stimulated rat pituitary cells. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8540–8544. doi: 10.1073/pnas.83.22.8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens P. M., De Jong C. C., Vink A. A., Van Haastert P. J. Regulatory properties of magnesium-dependent guanylate cyclase in Dictyostelium discoideum membranes. J Biol Chem. 1989 Mar 15;264(8):4329–4335. [PubMed] [Google Scholar]
- Johnson R. L., Gundersen R., Lilly P., Pitt G. S., Pupillo M., Sun T. J., Vaughan R. A., Devreotes P. N. G-protein-linked signal transduction systems control development in Dictyostelium. Development. 1989;107 (Suppl):75–80. doi: 10.1242/dev.107.Supplement.75. [DOI] [PubMed] [Google Scholar]
- Kesbeke F., Snaar-Jagalska B. E., Van Haastert P. J. Signal transduction in Dictyostelium fgd A mutants with a defective interaction between surface cAMP receptors and a GTP-binding regulatory protein. J Cell Biol. 1988 Aug;107(2):521–528. doi: 10.1083/jcb.107.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel A. R., Firtel R. A. cAMP signal transduction pathways regulating development of Dictyostelium discoideum. Curr Opin Genet Dev. 1991 Oct;1(3):383–390. doi: 10.1016/s0959-437x(05)80304-1. [DOI] [PubMed] [Google Scholar]
- Klein P. S., Sun T. J., Saxe C. L., 3rd, Kimmel A. R., Johnson R. L., Devreotes P. N. A chemoattractant receptor controls development in Dictyostelium discoideum. Science. 1988 Sep 16;241(4872):1467–1472. doi: 10.1126/science.3047871. [DOI] [PubMed] [Google Scholar]
- Kumagai A., Pupillo M., Gundersen R., Miake-Lye R., Devreotes P. N., Firtel R. A. Regulation and function of G alpha protein subunits in Dictyostelium. Cell. 1989 Apr 21;57(2):265–275. doi: 10.1016/0092-8674(89)90964-1. [DOI] [PubMed] [Google Scholar]
- Leslie C. C. Kinetic properties of a high molecular mass arachidonoyl-hydrolyzing phospholipase A2 that exhibits lysophospholipase activity. J Biol Chem. 1991 Jun 15;266(17):11366–11371. [PubMed] [Google Scholar]
- Long B. H., Coe E. L. Changes in neutral lipid constituents during differentiation of the cellular slime mold, Dictyostelium discoideum. J Biol Chem. 1974 Jan 25;249(2):521–529. [PubMed] [Google Scholar]
- Lundberg G. A., Newell P. C. Membrane-associated phosphoinositidase C activity in Dictyostelium discoideum. FEBS Lett. 1990 Sep 17;270(1-2):181–183. doi: 10.1016/0014-5793(90)81262-m. [DOI] [PubMed] [Google Scholar]
- MacDonald J. I., Weeks G. The biosynthesis and turnover of lipid during the differentiation of Dictyostelium discoideum. Biochim Biophys Acta. 1985 May 17;834(3):301–307. doi: 10.1016/0005-2760(85)90002-5. [DOI] [PubMed] [Google Scholar]
- Mehdy M. C., Ratner D., Firtel R. A. Induction and modulation of cell-type-specific gene expression in Dictyostelium. Cell. 1983 Mar;32(3):763–771. doi: 10.1016/0092-8674(83)90062-4. [DOI] [PubMed] [Google Scholar]
- Milne J. L., Coukell M. B. A Ca2+ transport system associated with the plasma membrane of Dictyostelium discoideum is activated by different chemoattractant receptors. J Cell Biol. 1991 Jan;112(1):103–110. doi: 10.1083/jcb.112.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell P. C., Europe-Finner G. N., Liu G., Gammon B., Wood C. A. Signal transduction for chemotaxis in Dictyostelium amoebae. Semin Cell Biol. 1990 Apr;1(2):105–113. [PubMed] [Google Scholar]
- Pitt G. S., Gundersen R. E., Lilly P. J., Pupillo M. B., Vaughan R. A., Devreotes P. N. G protein-linked signal transduction in aggregating Dictyostelium. Soc Gen Physiol Ser. 1990;45:125–131. [PubMed] [Google Scholar]
- Rhee S. G., Suh P. G., Ryu S. H., Lee S. Y. Studies of inositol phospholipid-specific phospholipase C. Science. 1989 May 5;244(4904):546–550. doi: 10.1126/science.2541501. [DOI] [PubMed] [Google Scholar]
- Saxe C. L., 3rd, Johnson R. L., Devreotes P. N., Kimmel A. R. Expression of a cAMP receptor gene of Dictyostelium and evidence for a multigene family. Genes Dev. 1991 Jan;5(1):1–8. doi: 10.1101/gad.5.1.1. [DOI] [PubMed] [Google Scholar]
- Stephens L. R., Irvine R. F. Stepwise phosphorylation of myo-inositol leading to myo-inositol hexakisphosphate in Dictyostelium. Nature. 1990 Aug 9;346(6284):580–583. doi: 10.1038/346580a0. [DOI] [PubMed] [Google Scholar]
- Stephens L. R., Kay R. R., Irvine R. F. A myo-inositol D-3 hydroxykinase activity in Dictyostelium. Biochem J. 1990 Nov 15;272(1):201–210. doi: 10.1042/bj2720201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockbridge N. EGTA. Comput Biol Med. 1987;17(5):299–304. doi: 10.1016/0010-4825(87)90019-9. [DOI] [PubMed] [Google Scholar]
- Sun T. J., Van Haastert P. J., Devreotes P. N. Surface cAMP receptors mediate multiple responses during development in Dictyostelium: evidenced by antisense mutagenesis. J Cell Biol. 1990 May;110(5):1549–1554. doi: 10.1083/jcb.110.5.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. W. The role of G proteins in transmembrane signalling. Biochem J. 1990 Nov 15;272(1):1–13. doi: 10.1042/bj2720001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theibert A., Palmisano M., Jastorff B., Devreotes P. The specificity of the cAMP receptor mediating activation of adenylate cyclase in Dictyostelium discoideum. Dev Biol. 1986 Apr;114(2):529–533. doi: 10.1016/0012-1606(86)90216-2. [DOI] [PubMed] [Google Scholar]
- Tompkins T. A., Moscarello M. A. A 57-kDa phosphatidylinositol-specific phospholipase C from bovine brain. J Biol Chem. 1991 Mar 5;266(7):4228–4236. [PubMed] [Google Scholar]
- Van Haastert P. J., De Vries M. J., Penning L. C., Roovers E., Van der Kaay J., Erneux C., Van Lookeren Campagne M. M. Chemoattractant and guanosine 5'-[gamma-thio]triphosphate induce the accumulation of inositol 1,4,5-trisphosphate in Dictyostelium cells that are labelled with [3H]inositol by electroporation. Biochem J. 1989 Mar 1;258(2):577–586. doi: 10.1042/bj2580577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haastert P. J. Down-regulation of cell surface cyclic AMP receptors and desensitization of cyclic AMP-stimulated adenylate cyclase by cyclic AMP in Dictyostelium discoideum. Kinetics and concentration dependence. J Biol Chem. 1987 Jun 5;262(16):7700–7704. [PubMed] [Google Scholar]
- Van Lookeren Campagne M. M., Erneux C., Van Eijk R., Van Haastert P. J. Two dephosphorylation pathways of inositol 1,4,5-trisphosphate in homogenates of the cellular slime mould Dictyostelium discoideum. Biochem J. 1988 Sep 1;254(2):343–350. doi: 10.1042/bj2540343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks G. The manipulation of the fatty acid composition of Dictyostelium discoideum and its effect on cell differentiation. Biochim Biophys Acta. 1976 Oct 21;450(1):21–32. doi: 10.1016/0005-2760(76)90295-2. [DOI] [PubMed] [Google Scholar]
- van Blitterswijk W. J., Hilkmann H., de Widt J., van der Bend R. L. Phospholipid metabolism in bradykinin-stimulated human fibroblasts. II. Phosphatidylcholine breakdown by phospholipases C and D; involvement of protein kinase C. J Biol Chem. 1991 Jun 5;266(16):10344–10350. [PubMed] [Google Scholar]
- van Haastert P. J., de Wit R. J., Janssens P. M., Kesbeke F., DeGoede J. G-protein-mediated interconversions of cell-surface cAMP receptors and their involvement in excitation and desensitization of guanylate cyclase in Dictyostelium discoideum. J Biol Chem. 1986 May 25;261(15):6904–6911. [PubMed] [Google Scholar]


