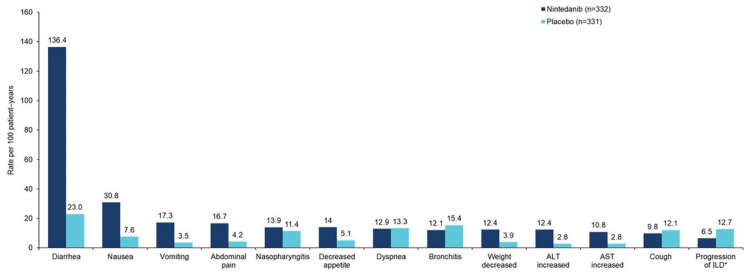
Figure 4.
Most frequent adverse events reported in the INBUILD trial. 18
Data are based on adverse events reported between the first trial drug intake and 28 days after the last trial drug intake. The median exposure to the trial drug was 17.4 months in both groups. Adverse events were coded based on single preferred terms in the Medical Dictionary for Regulatory Activities (MedDRA) version 22.0, except for abdominal pain, which was based on a group of MedDRA-preferred terms. Adverse events with a rate >10 events per 100 patient-years in either treatment group are shown.
*Based on MedDRA preferred term interstitial lung disease.
ALT, alanine aminotransferase; AST, aspartate aminotransferase.