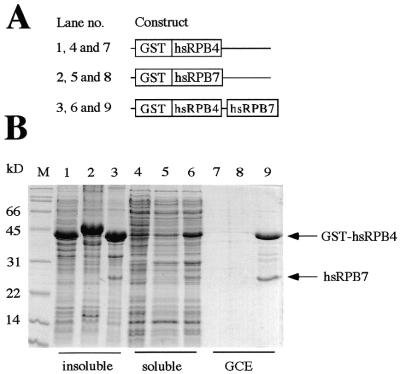
Figure 2.
Coexpression of GST–hsRPB4 and hsRPB7 subunits in E.coli. (A) Schematic representation of the expression constructs used. (B) Comparison of the protein expression profiles of bacterial cells harboring the constructs illustrated above. The insoluble and soluble fractions, together with the glutathione column eluates (GCE), are shown for the GST–hsRPB4 (lanes 1, 4 and 7), GST–hsRPB7 (lanes 2, 5 and 8) and GST–hsRPB4/hsRPB7 (lanes 3, 6 and 9) expression strains. The GST–hsRPB4 and GST–hsRPB7 fusion proteins are highly insoluble when expressed by themselves and are thus exclusively present in the pellet fractions (lanes 1 and 2). The bicistronic construct expressing GST–hRPB4 and hRPB7 leads, however, to the formation of a heterodimeric complex with increased solubility that can be purified by glutathione affinity chromatography (lane 9).