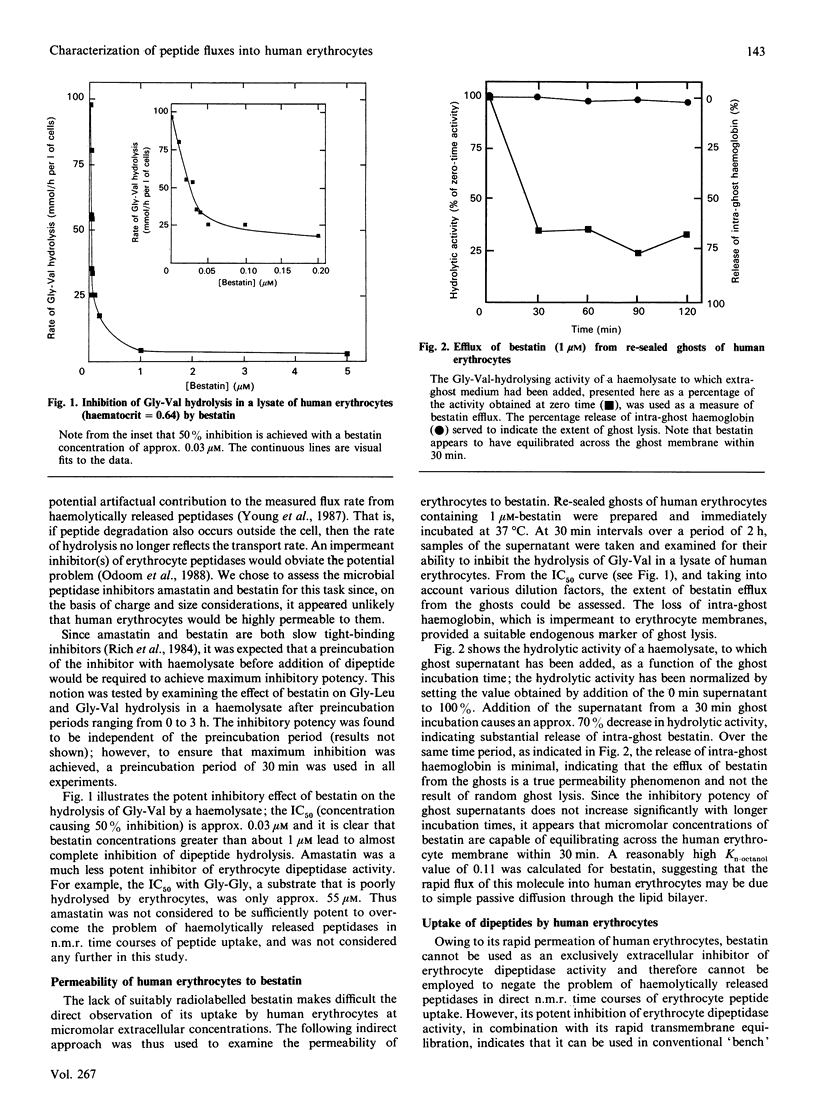
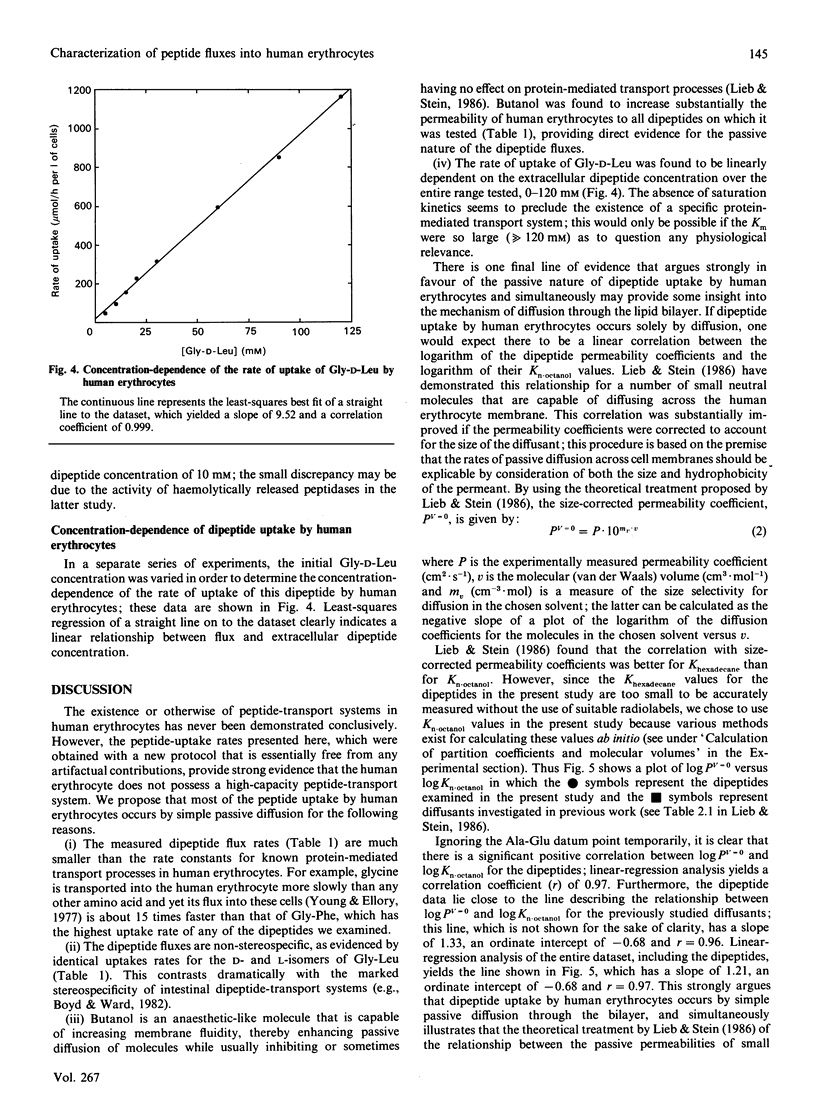
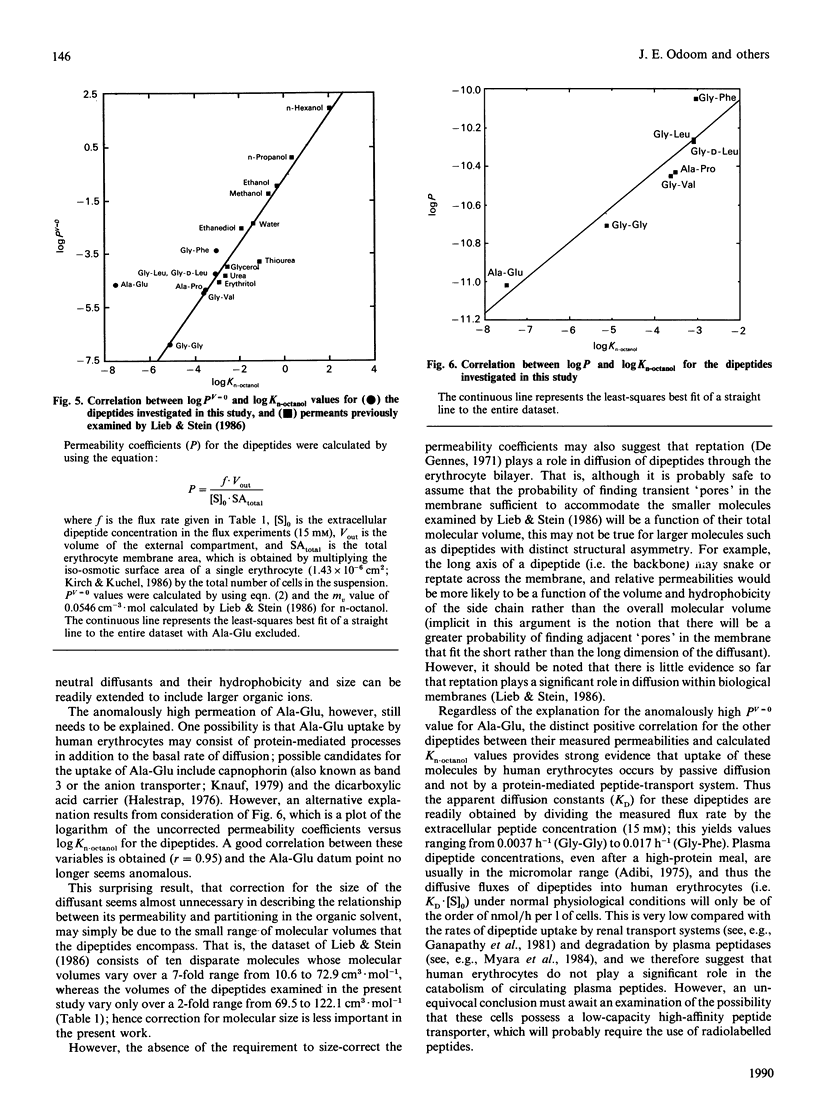
Abstract
A new protocol for measuring cellular uptake of dipeptides was developed in which the problem of peptide hydrolysis is obviated by introduction into the cell suspension of a membrane-permeant peptidase inhibitor. The uptake of unlabelled dipeptide is readily monitored so long as some analytical technique is available for measuring the intracellular peptide concentration; in this study we used n.m.r. spectroscopy. Using this protocol, we demonstrated that dipeptide uptake by human erythrocytes occurs by simple diffusion through the lipid bilayer and not via a high-capacity protein-mediated transport system. Substantiating evidence includes demonstration that: (a) the fluxes are slow compared with known protein-mediated transport processes in human erythrocytes; (b) the uptake is not stereospecific; (c) the uptake does not display saturation kinetics; (d) the fluxes are significantly enhanced by butanol; (e) a distinct correlation exists between the size-corrected permeability coefficients of the dipeptides and their calculated n-octanol/water partition coefficients. It is calculated that under normal physiological conditions the diffusive fluxes of circulating plasma peptides into human erythrocytes are too small for these cells to play a significant role in dipeptide catabolism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beilharz G. R., Middlehurst C. R., Kuchel P. W., Hunt G. E., Johnson G. F. Determination of choline in erythrocytes using high-resolution proton nuclear magnetic resonance spectroscopy: comparison with a choline oxidase method. Anal Biochem. 1984 Mar;137(2):324–329. doi: 10.1016/0003-2697(84)90093-9. [DOI] [PubMed] [Google Scholar]
- Beutler E., West C., Blume K. G. The removal of leukocytes and platelets from whole blood. J Lab Clin Med. 1976 Aug;88(2):328–333. [PubMed] [Google Scholar]
- Boyd C. A., Ward M. R. A micro-electrode study of oligopeptide absorption by the small intestinal epithelium of Necturus maculosus. J Physiol. 1982 Mar;324:411–428. doi: 10.1113/jphysiol.1982.sp014121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown F. F., Campbell I. D., Kuchel P. W., Rabenstein D. C. Human erythrocyte metabolism studies by 1H spin echo NMR. FEBS Lett. 1977 Oct 1;82(1):12–16. doi: 10.1016/0014-5793(77)80875-2. [DOI] [PubMed] [Google Scholar]
- Endo F., Matsuda I., Ogata A., Tanaka S. Human erythrocyte prolidase and prolidase deficiency. Pediatr Res. 1982 Mar;16(3):227–231. doi: 10.1203/00006450-198203000-00013. [DOI] [PubMed] [Google Scholar]
- Ganapathy M. E., Mahesh V. B., Devoe L. D., Leibach F. H., Ganapathy V. Dipeptide transport in brush-border membrane vesicles isolated from normal term human placenta. Am J Obstet Gynecol. 1985 Sep 1;153(1):83–86. doi: 10.1016/0002-9378(85)90600-3. [DOI] [PubMed] [Google Scholar]
- Ganapathy V., Leibach F. H. Carrier-mediated reabsorption of small peptides in renal proximal tubule. Am J Physiol. 1986 Dec;251(6 Pt 2):F945–F953. doi: 10.1152/ajprenal.1986.251.6.F945. [DOI] [PubMed] [Google Scholar]
- Ganapathy V., Mendicino J., Leibach F. H. Evidence for a dipeptide transport system in renal brush border membranes from rabbit. Biochim Biophys Acta. 1981 Apr 6;642(2):381–391. doi: 10.1016/0005-2736(81)90454-5. [DOI] [PubMed] [Google Scholar]
- Halestrap A. P. Transport of pyruvate nad lactate into human erythrocytes. Evidence for the involvement of the chloride carrier and a chloride-independent carrier. Biochem J. 1976 May 15;156(2):193–207. doi: 10.1042/bj1560193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isab A. A., Rabenstein D. L. The incorporation of 2H-labelled glycine into the glutathione of intact human erythrocytes studied by 1H spin-echo Fourier transform NMR. FEBS Lett. 1979 Oct 15;106(2):325–329. doi: 10.1016/0014-5793(79)80525-6. [DOI] [PubMed] [Google Scholar]
- King G. F., Crossley M. J., Kuchel P. W. Inhibition and active-site modelling of prolidase. Eur J Biochem. 1989 Mar 15;180(2):377–384. doi: 10.1111/j.1432-1033.1989.tb14659.x. [DOI] [PubMed] [Google Scholar]
- King G. F., Kuchel P. W. A proton n.m.r. study of iminodipeptide transport and hydrolysis in the human erythrocyte. Possible physiological roles for the coupled system. Biochem J. 1984 Jun 1;220(2):553–560. doi: 10.1042/bj2200553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G. F., Kuchel P. W. Assimilation of alpha-glutamyl-peptides by human erythrocytes. A possible means of glutamate supply for glutathione synthesis. Biochem J. 1985 May 1;227(3):833–842. doi: 10.1042/bj2270833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G. F., Middlehurst C. R., Kuchel P. W. Direct NMR evidence that prolidase is specific for the trans isomer of imidodipeptide substrates. Biochemistry. 1986 Mar 11;25(5):1054–1062. doi: 10.1021/bi00353a016. [DOI] [PubMed] [Google Scholar]
- King G. F., York M. J., Chapman B. E., Kuchel P. W. Proton NMR spectroscopic studies of dipeptidase in human erythrocytes. Biochem Biophys Res Commun. 1983 Jan 14;110(1):305–312. doi: 10.1016/0006-291x(83)91296-2. [DOI] [PubMed] [Google Scholar]
- Kuchel P. W., King G. F., Chapman B. E. No evidence of high capacity alpha-glutamyl-dipeptide transport into human erythrocytes. Biochem J. 1987 Feb 15;242(1):311–312. doi: 10.1042/bj2420311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myara I., Myara A., Mangeot M., Fabre M., Charpentier C., Lemonnier A. Plasma prolidase activity: a possible index of collagen catabolism in chronic liver disease. Clin Chem. 1984 Feb;30(2):211–215. [PubMed] [Google Scholar]
- Rich D. H., Moon B. J., Harbeson S. Inhibition of aminopeptidases by amastatin and bestatin derivatives. Effect of inhibitor structure on slow-binding processes. J Med Chem. 1984 Apr;27(4):417–422. doi: 10.1021/jm00370a001. [DOI] [PubMed] [Google Scholar]
- Young D. J., Wolowyk M. W., Fincham D. A., Cheeseman C. I., Rabenstein D. L., Ellory J. C. Conflicting evidence regarding the transport of alpha-glutamyl-dipeptides by human erythrocytes. Biochem J. 1987 Feb 15;242(1):309–311. doi: 10.1042/bj2420309. [DOI] [PMC free article] [PubMed] [Google Scholar]


