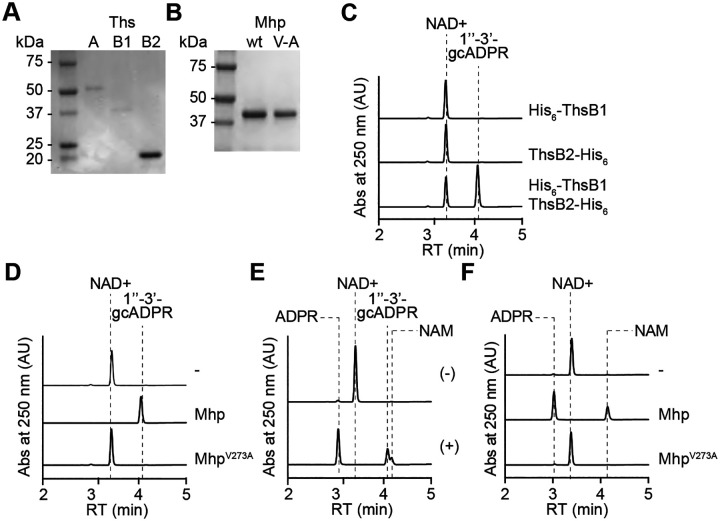
Figure 5. Viral major head protein is sufficient to stimulate ThsB1/B2 cyclase activity in vitro.
(A) Coomassie Blue-stained SDS-PAGE of proteins purified from E. coli expressing His6-ThsA (56.0 kDa), His6-ThsB1 (41.8 kDa), and ThsB2-His6 (23.4 kDa) using a cobalt resin. Protein molecular weight (kDa) markers are shown. (B) Coomassie Blue-stained SDS-PAGE of Mhp proteins, wild-type and V273A mutant (V/A) purified from staphylococci harboring pMhp plasmids, using PEG-enrichment. Protein molecular weight (kDa) markers are shown. (C) HPLC analysis of the products resulting from the incubation of purified ThsB2-His6, ThsB2F6A-His6 or both, with NAD+. Retention times (RT) of reactants and products are marked by dotted lines. (D) Same as (C) but after incubation of both ThsB2-His6 and ThsB2F6A-His6, alone (−) or in the presence of purified wild-type or V273A mutant Mhp. (E) HPLC analysis of the products resulting from the incubation of purified His6-ThsA and commercially available 1”-3’ gcADPR. Retention times (RT) of reactants and products are marked by dotted lines. (F) Same as (D) but in the presence of purified His6-ThsA.