Abstract
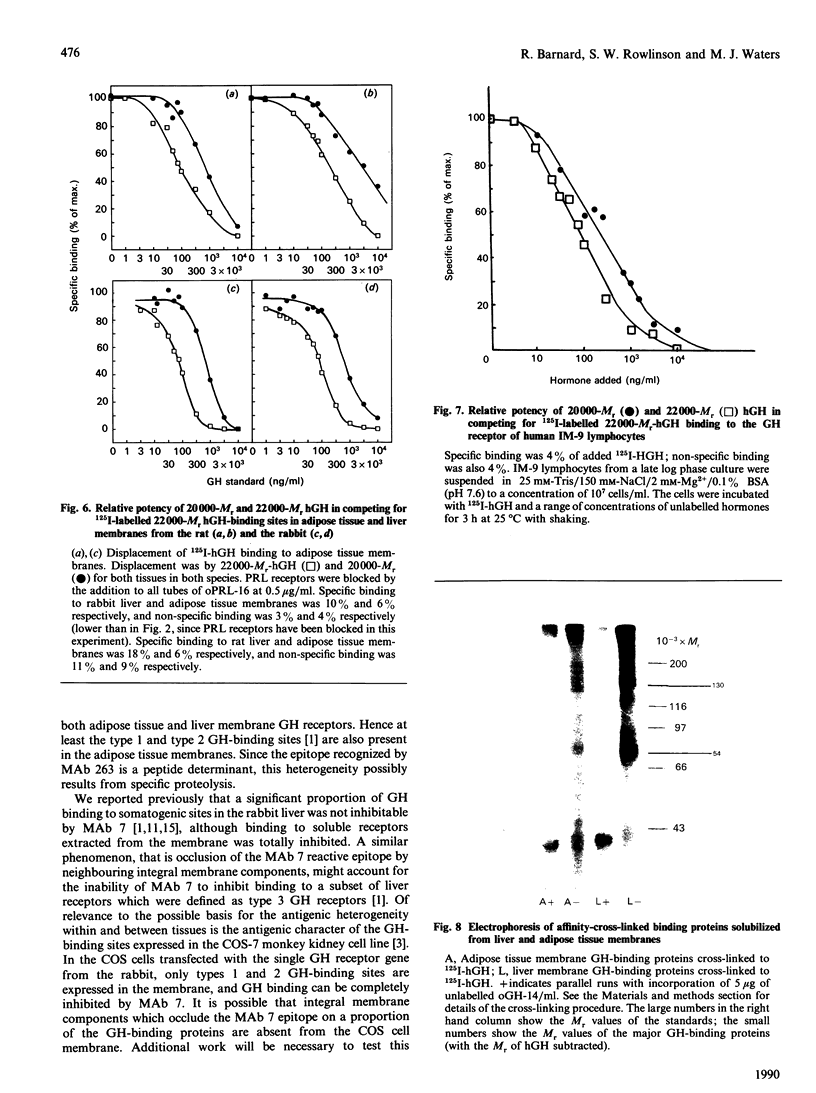
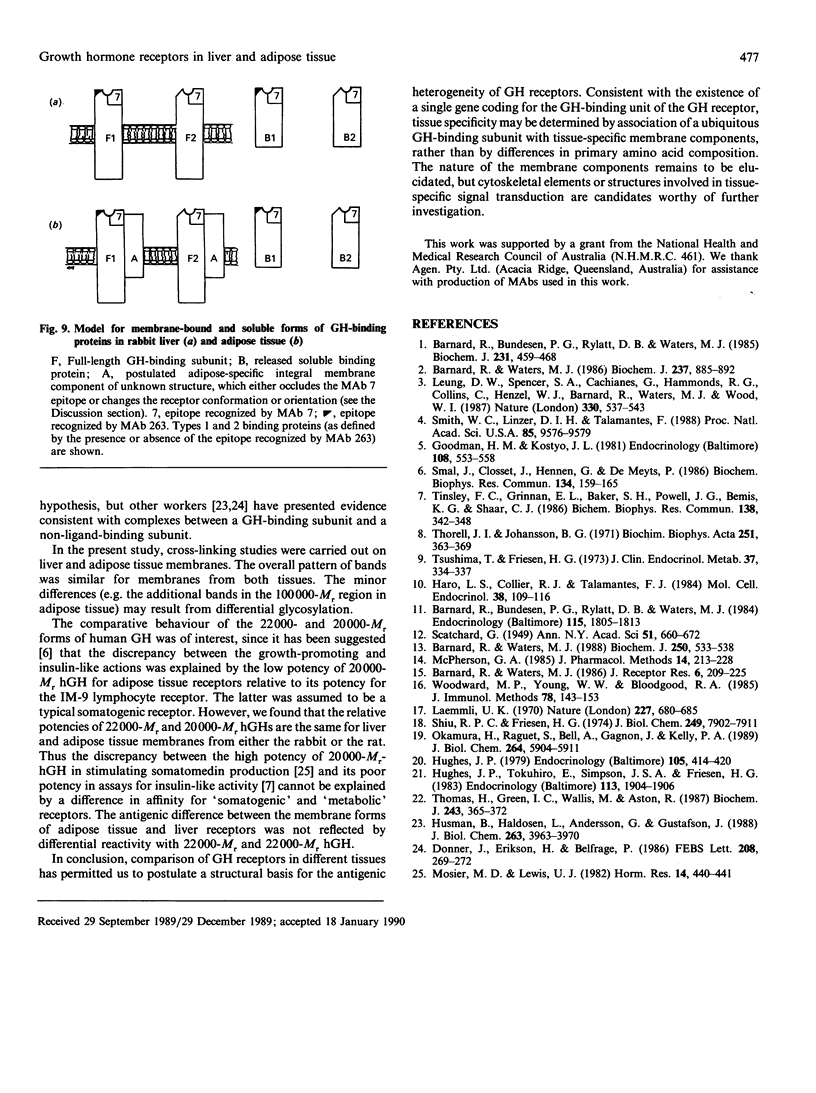
Cytosolic, detergent-solubilized and membrane-bound growth hormone (GH) receptors from rabbit adipose tissue and liver were tested for reactivity with a panel of monoclonal antibodies (MAbs). The cytosolic and detergent-solubilized forms of adipose tissue and liver GH receptors were identically reactive with four precipitating and two hormone-binding-site-directed MAbs. However, the membrane-bound form of the adipose receptor was 1000-fold less reactive with one binding-site-directed MAb (MAb 7) than the membrane-bound liver GH receptor. Reactivity with another inhibitory MAb (MAb 263) was identical for adipose tissue and liver membrane GH receptors. The relative potency of 22,000-Mr and 20,000-Mr forms of human GH was identical in assays with liver and adipose tissue membrane receptors. Thus, contrary to earlier suggestions, the discrepancy between the growth-promoting and insulin-like activities of 20,000-Mr human GH cannot be rationalized by a difference in the affinity of this hormone for 'somatogenic' and 'metabolic' receptors when the comparison is made in the same species. Cross-linking studies showed that the major GH-binding subunit of liver and adipose tissue GH receptors had the same Mr (54,000 +/- 5000, reduced). The ligand-binding subunits of liver and adipose tissue receptors are identical by several criteria, but one epitope on the adipose tissue receptor appears to be masked upon membrane insertion, possibly by close association with a tissue-specific component. Tissue specificity may be determined by association of a ubiquitous GH-binding subunit with tissue-specific membrane components, rather than by differences in amino acid sequence.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnard R., Bundesen P. G., Rylatt D. B., Waters M. J. Evidence from the use of monoclonal antibody probes for structural heterogeneity of the growth hormone receptor. Biochem J. 1985 Oct 15;231(2):459–468. doi: 10.1042/bj2310459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard R., Bundesen P. G., Rylatt D. B., Waters M. J. Monoclonal antibodies to the rabbit liver growth hormone receptor: production and characterization. Endocrinology. 1984 Nov;115(5):1805–1813. doi: 10.1210/endo-115-5-1805. [DOI] [PubMed] [Google Scholar]
- Barnard R., Waters M. J. Evidence for differential binding of growth hormones to membrane and cytosolic GH binding proteins of rabbit liver. J Recept Res. 1986;6(3-4):209–225. doi: 10.3109/10799898609074811. [DOI] [PubMed] [Google Scholar]
- Barnard R., Waters M. J. Serum and liver cytosolic growth-hormone-binding proteins are antigenically identical with liver membrane 'receptor' types 1 and 2. Biochem J. 1986 Aug 1;237(3):885–892. doi: 10.1042/bj2370885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard R., Waters M. J. Use of calcium dependence as a means to study the interaction between growth hormones and their binding proteins in rabbit liver. Biochem J. 1988 Mar 1;250(2):533–538. doi: 10.1042/bj2500533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnér J., Eriksson H., Belfrage P. The acute GH action in rat adipocytes is associated with enhanced phosphorylation of a 46 kDa plasma membrane protein enriched by GH-Sepharose. FEBS Lett. 1986 Nov 24;208(2):269–272. doi: 10.1016/0014-5793(86)81030-4. [DOI] [PubMed] [Google Scholar]
- Goodman H. M., Kostyo J. L. Altered profiles of biological activity of growth hormone fragments on adipocyte metabolism. Endocrinology. 1981 Feb;108(2):553–558. doi: 10.1210/endo-108-2-553. [DOI] [PubMed] [Google Scholar]
- Haro L. S., Collier R. J., Talamantes F. J. Homologous somatotropin radioreceptor assay utilizing recombinant bovine growth hormone. Mol Cell Endocrinol. 1984 Dec;38(2-3):109–116. doi: 10.1016/0303-7207(84)90109-6. [DOI] [PubMed] [Google Scholar]
- Hughes J. P. Identification and characterization of high and low affinity binding sites for growth hormone in rabbit liver. Endocrinology. 1979 Aug;105(2):414–420. doi: 10.1210/endo-105-2-414. [DOI] [PubMed] [Google Scholar]
- Hughes J. P., Tokuhiro E., Simpson J. S., Friesen H. G. 20K is bound with high affinity by one rat and one of two rabbit growth hormone receptors. Endocrinology. 1983 Nov;113(5):1904–1906. doi: 10.1210/endo-113-5-1904. [DOI] [PubMed] [Google Scholar]
- Husman B., Haldosén L. A., Andersson G., Gustafsson J. A. Characterization of the somatogenic receptor in rat liver. Hydrodynamic properties and affinity cross-linking. J Biol Chem. 1988 Mar 15;263(8):3963–3970. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leung D. W., Spencer S. A., Cachianes G., Hammonds R. G., Collins C., Henzel W. J., Barnard R., Waters M. J., Wood W. I. Growth hormone receptor and serum binding protein: purification, cloning and expression. Nature. 1987 Dec 10;330(6148):537–543. doi: 10.1038/330537a0. [DOI] [PubMed] [Google Scholar]
- McPherson G. A. Analysis of radioligand binding experiments. A collection of computer programs for the IBM PC. J Pharmacol Methods. 1985 Nov;14(3):213–228. doi: 10.1016/0160-5402(85)90034-8. [DOI] [PubMed] [Google Scholar]
- Mosier H. D., Jr, Lewis U. J. The 20,000-dalton variant of human growth hormone: effect on bioassayable somatomedin activity in serum. Horm Metab Res. 1982 Aug;14(8):440–441. doi: 10.1055/s-2007-1019040. [DOI] [PubMed] [Google Scholar]
- Okamura H., Raguet S., Bell A., Gagnon J., Kelly P. A. Purification and protein sequence analysis of rat liver prolactin receptor. J Biol Chem. 1989 Apr 5;264(10):5904–5911. [PubMed] [Google Scholar]
- Shiu R. P., Friesen H. G. Solubilization and purification of a prolactin receptor from the rabbit mammary gland. J Biol Chem. 1974 Dec 25;249(24):7902–7911. [PubMed] [Google Scholar]
- Smal J., Closset J., Hennen G., De Meyts P. The receptor binding properties of the 20K variant of human growth hormone explain its discrepant insulin-like and growth promoting activities. Biochem Biophys Res Commun. 1986 Jan 14;134(1):159–165. doi: 10.1016/0006-291x(86)90541-3. [DOI] [PubMed] [Google Scholar]
- Smith W. C., Linzer D. I., Talamantes F. Detection of two growth hormone receptor mRNAs and primary translation products in the mouse. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9576–9579. doi: 10.1073/pnas.85.24.9576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas H., Green I. C., Wallis M., Aston R. Heterogeneity of growth-hormone receptors detected with monoclonal antibodies to human growth hormone. Biochem J. 1987 Apr 15;243(2):365–372. doi: 10.1042/bj2430365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorell J. I., Johansson B. G. Enzymatic iodination of polypeptides with 125I to high specific activity. Biochim Biophys Acta. 1971 Dec 28;251(3):363–369. doi: 10.1016/0005-2795(71)90123-1. [DOI] [PubMed] [Google Scholar]
- Tinsley F. C., Grinnan E. L., Baker S. H., Powell J. G., Bemis K. G., Shaar C. J. The 20,000 dalton structural variant of recombinant DNA-derived methionyl human growth hormone has early insulin-like effects in hypophysectomized rats. Biochem Biophys Res Commun. 1986 Jul 16;138(1):342–348. doi: 10.1016/0006-291x(86)90286-x. [DOI] [PubMed] [Google Scholar]
- Tsushima T., Friesen H. G. Radioreceptor assay for growth hormone. J Clin Endocrinol Metab. 1973 Aug;37(2):334–337. doi: 10.1210/jcem-37-2-334. [DOI] [PubMed] [Google Scholar]
- Woodward M. P., Young W. W., Jr, Bloodgood R. A. Detection of monoclonal antibodies specific for carbohydrate epitopes using periodate oxidation. J Immunol Methods. 1985 Apr 8;78(1):143–153. doi: 10.1016/0022-1759(85)90337-0. [DOI] [PubMed] [Google Scholar]