Abstract
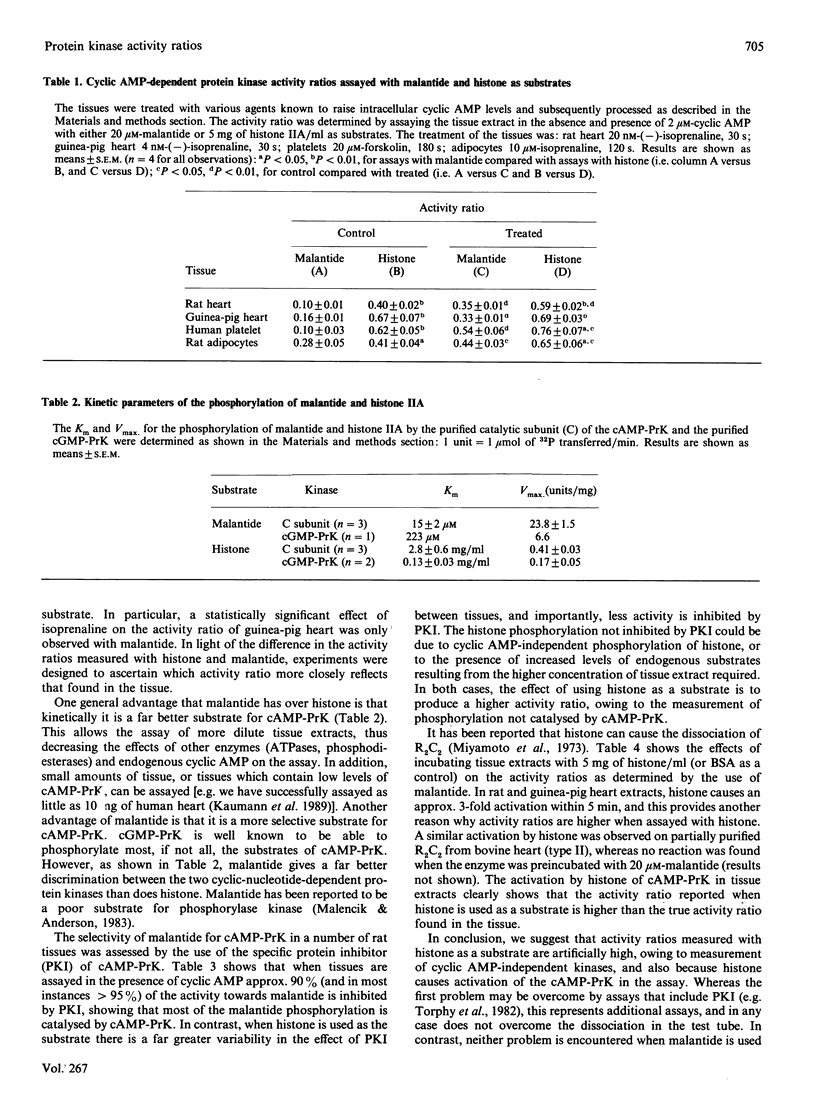
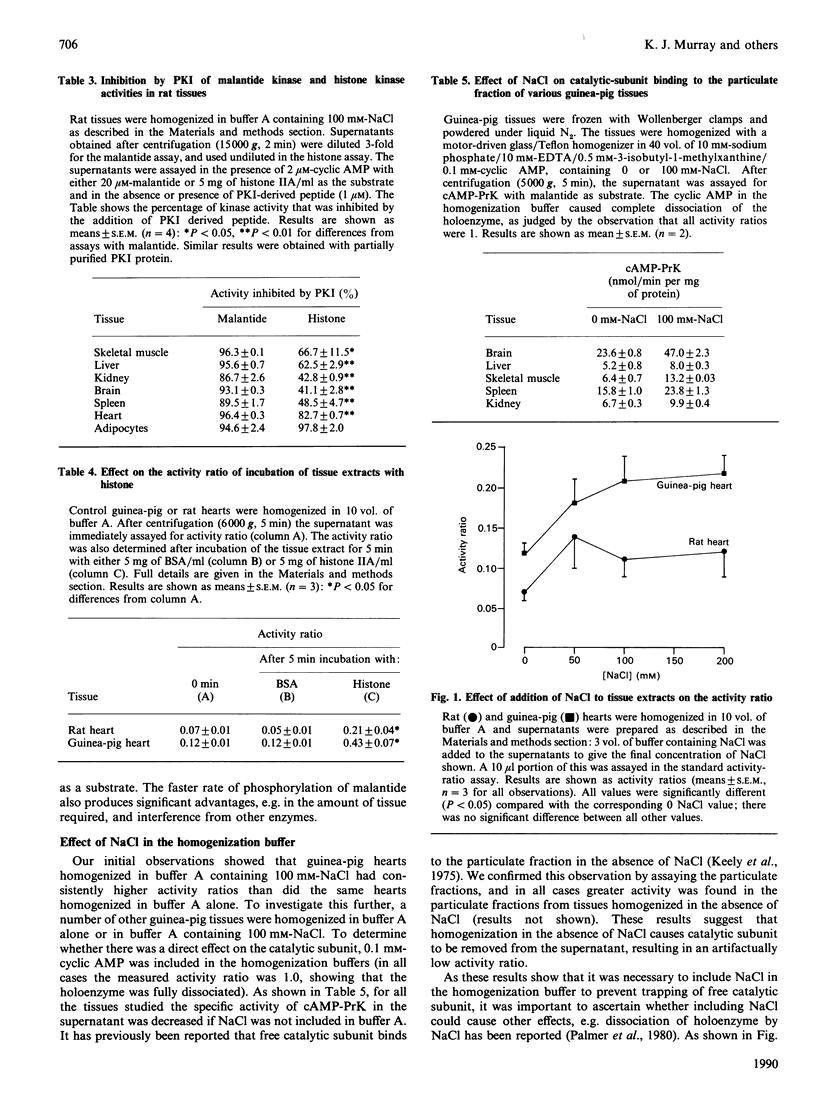
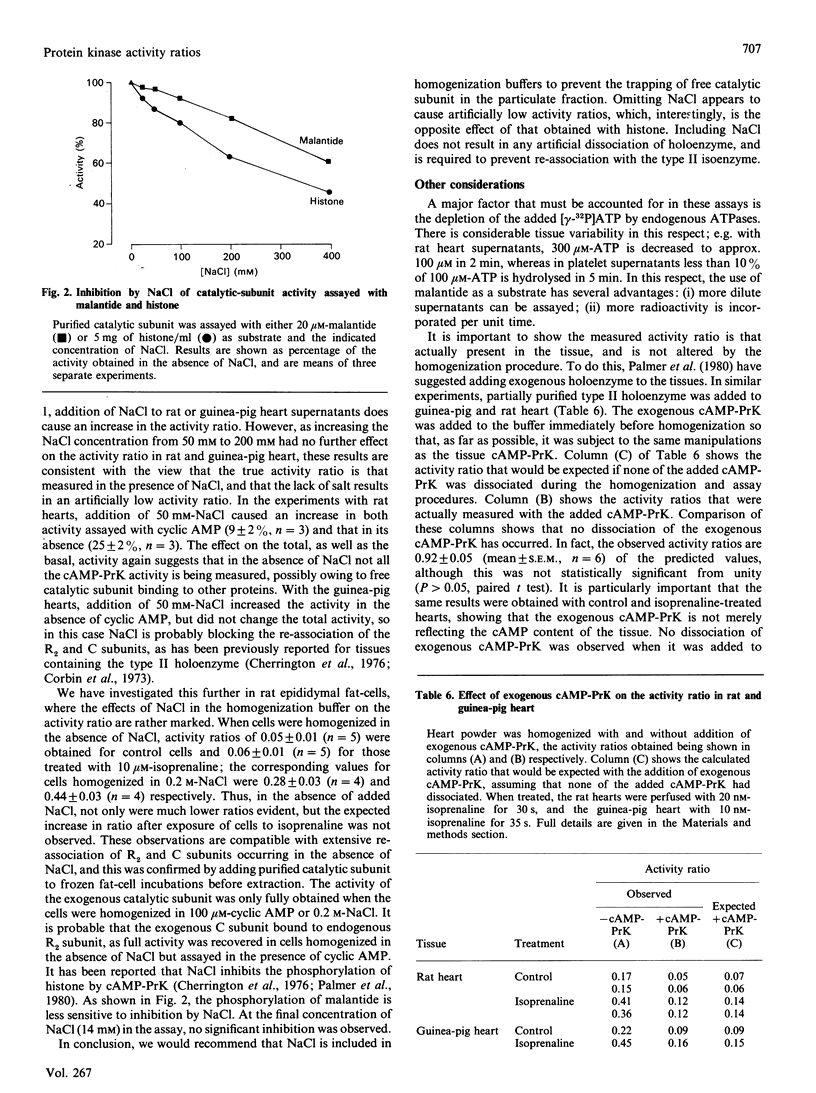
1. The cyclic AMP-dependent protein kinase activity-ratio assay was investigated by comparing histone and a synthetic peptide, malantide [Malencik & Anderson (1983) Anal. Biochem. 132, 32-40], as substrates. 2. In several tissues the activity ratio was higher when assayed with histone as the substrate; this result was obtained in control tissues and also in those incubated with agents known to increase cyclic AMP. The effect of these agents to increase the activity ratio was more clearly demonstrated with malantide. 3. The higher activity ratios observed with histone are due to: (a) measurement of phosphorylation not catalysed by cyclic AMP-dependent protein kinase; (b) activation of cyclic AMP-dependent protein kinase by histone during the assay. 4. When tissues were homogenized in buffers without NACl, lower activity ratios were found, owing to the catalytic subunit being artifactually removed from the supernatant. 5. We conclude that the measured activity ratio more faithfully reflects that in the tissue when NaCl is included in the homogenization buffer and malantide is used in the assay. This was confirmed in experiments where cyclic AMP-dependent protein kinase was added to the tissue before homogenization, and no dissociation of the exogenous enzyme was observed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cheng H. C., Kemp B. E., Pearson R. B., Smith A. J., Misconi L., Van Patten S. M., Walsh D. A. A potent synthetic peptide inhibitor of the cAMP-dependent protein kinase. J Biol Chem. 1986 Jan 25;261(3):989–992. [PubMed] [Google Scholar]
- Cherrington A. D., Assimacopoulos F. D., Harper S. C., Corbin J. D., Park C. R., Exton J. H. Studies on the alpha-andrenergic activation of hepatic glucose output. II. Investigation of the roles of adenosine 3':5'-monophosphate and adenosine 3':5'-monophosphate-dependent protein kinase in the actions of phenylephrine in isolated hepatocytes. J Biol Chem. 1976 Sep 10;251(17):5209–5218. [PubMed] [Google Scholar]
- Corbin J. D. Determination of the cAMP-dependent protein kinase activity ratio in intact tissues. Methods Enzymol. 1983;99:227–232. doi: 10.1016/0076-6879(83)99057-2. [DOI] [PubMed] [Google Scholar]
- Corbin J. D., Reimann E. M. Assay of cyclic AMP-dependent protein kinases. Methods Enzymol. 1974;38:287–290. doi: 10.1016/0076-6879(74)38044-5. [DOI] [PubMed] [Google Scholar]
- Corbin J. D., Soderling T. R., Park C. R. Regulation of adenosine 3',5'-monophosphate-dependent protein kinase. I. Preliminary characterization of the adipose tissue enzyme in crude extracts. J Biol Chem. 1973 Mar 10;248(5):1813–1821. [PubMed] [Google Scholar]
- Corbin J. D., Sugden P. H., West L., Flockhart D. A., Lincoln T. M., McCarthy D. Studies on the properties and mode of action of the purified regulatory subunit of bovine heart adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1978 Jun 10;253(11):3997–4003. [PubMed] [Google Scholar]
- England P. J. Studies on the phosphorylation of the inhibitory subunit of troponin during modification of contraction in perfused rat heart. Biochem J. 1976 Nov 15;160(2):295–304. doi: 10.1042/bj1600295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giembycz M. A., Diamond J. Evaluation of kemptide, a synthetic serine-containing heptapeptide, as a phosphate acceptor for the estimation of cyclic AMP-dependent protein kinase activity in respiratory tissues. Biochem Pharmacol. 1990 Jan 15;39(2):271–283. doi: 10.1016/0006-2952(90)90026-h. [DOI] [PubMed] [Google Scholar]
- Honnor R. C., Dhillon G. S., Londos C. cAMP-dependent protein kinase and lipolysis in rat adipocytes. I. Cell preparation, manipulation, and predictability in behavior. J Biol Chem. 1985 Dec 5;260(28):15122–15129. [PubMed] [Google Scholar]
- Keely S. L., Jr, Corbin J. D., Park C. R. On the question of translocation of heart cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1501–1504. doi: 10.1073/pnas.72.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln T. M. cGMP-dependent protein kinase. Methods Enzymol. 1983;99:62–71. doi: 10.1016/0076-6879(83)99041-9. [DOI] [PubMed] [Google Scholar]
- Malencik D. A., Anderson S. R. Characterization of a fluorescent substrate for the adenosine 3',5'-cyclic monophosphate-dependent protein kinase. Anal Biochem. 1983 Jul 1;132(1):34–40. doi: 10.1016/0003-2697(83)90422-0. [DOI] [PubMed] [Google Scholar]
- Miyamoto E., Petzold G. L., Kuo J. F., Greengard P. Dissociation and activation of adenosine 3',5'-monophosphate-dependent and guanosine 3',5'-monophosphate-dependent protein kinases by cyclic nucleotides and by substrate proteins. J Biol Chem. 1973 Jan 10;248(1):179–189. [PubMed] [Google Scholar]
- Palmer W. K., McPherson J. M., Walsh D. A. Critical controls in the evaluation of cAMP-dependent protein kinase activity ratios as indices of hormonal action. J Biol Chem. 1980 Apr 10;255(7):2663–2666. [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Reimann E. M., Beham R. A. Catalytic subunit of cAMP-dependent protein kinase. Methods Enzymol. 1983;99:51–55. doi: 10.1016/0076-6879(83)99039-0. [DOI] [PubMed] [Google Scholar]
- SHUGAR D. The ultraviolet absorption spectrum of ribonuclease. Biochem J. 1952 Sep;52(1):142–149. doi: 10.1042/bj0520142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlender K. K., Tyma J. L., Reimann E. M. Preparation of partially purified protein kinase inhibitor. Methods Enzymol. 1983;99:77–80. doi: 10.1016/0076-6879(83)99043-2. [DOI] [PubMed] [Google Scholar]
- Seiler S., Arnold A. J., Grove R. I., Fifer C. A., Keely S. L., Jr, Stanton H. C. Effects of anagrelide on platelet cAMP levels, cAMP-dependent protein kinase and thrombin-induced Ca++ fluxes. J Pharmacol Exp Ther. 1987 Nov;243(2):767–774. [PubMed] [Google Scholar]
- Simpson A. W., Reeves M. L., Rink T. J. Effects of SK&F 94120, an inhibitor of cyclic nucleotide phosphodiesterase type III, on human platelets. Biochem Pharmacol. 1988 Jun 15;37(12):2315–2320. doi: 10.1016/0006-2952(88)90357-7. [DOI] [PubMed] [Google Scholar]
- Smith C. J., Manganiello V. C. Role of hormone-sensitive low Km cAMP phosphodiesterase in regulation of cAMP-dependent protein kinase and lipolysis in rat adipocytes. Mol Pharmacol. 1989 Mar;35(3):381–386. [PubMed] [Google Scholar]
- Torphy T. J., Freese W. B., Rinard G. A., Brunton L. L., Mayer S. E. Cyclic nucleotide-dependent protein kinases in airway smooth muscle. J Biol Chem. 1982 Oct 10;257(19):11609–11616. [PubMed] [Google Scholar]
- Whitesell R. R., Gliemann J. Kinetic parameters of transport of 3-O-methylglucose and glucose in adipocytes. J Biol Chem. 1979 Jun 25;254(12):5276–5283. [PubMed] [Google Scholar]
- Yeaman S. J., Cohen P., Watson D. C., Dixon G. H. The substrate specificity of adenosine 3':5'-cyclic monophosphate-dependent protein kinase of rabbit skeletal muscle. Biochem J. 1977 Feb 15;162(2):411–421. doi: 10.1042/bj1620411. [DOI] [PMC free article] [PubMed] [Google Scholar]


