Abstract
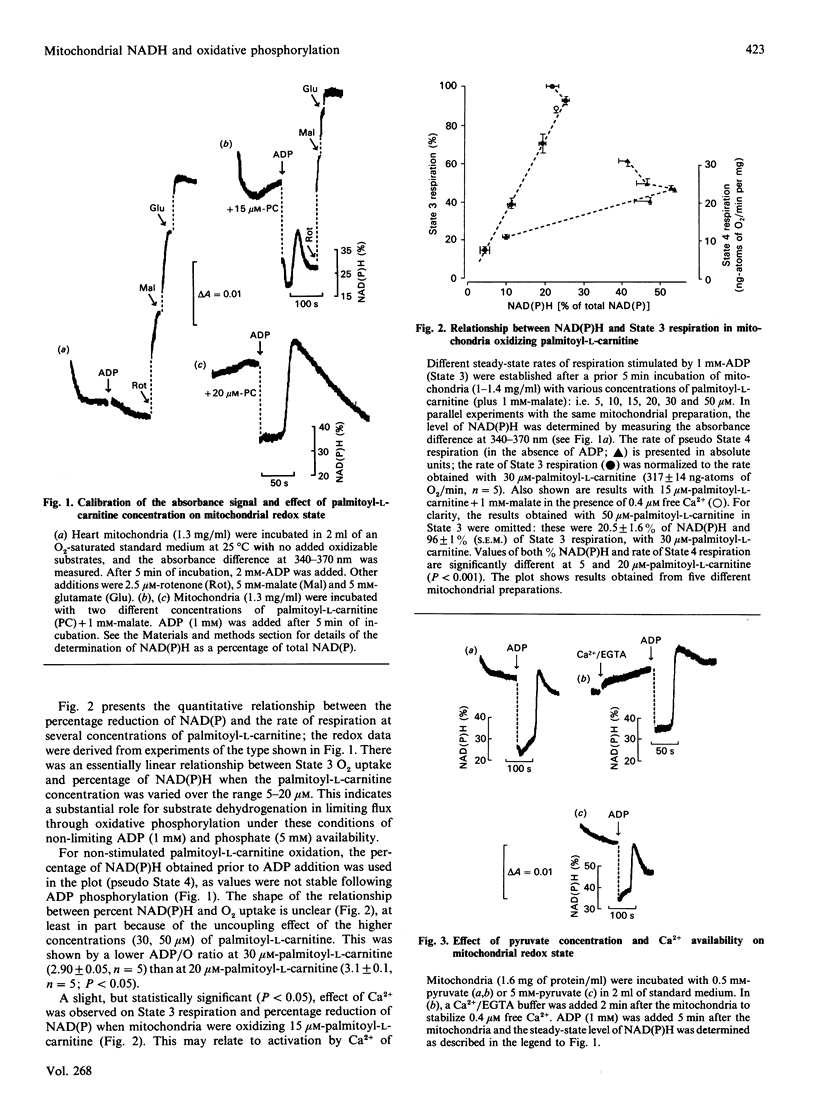
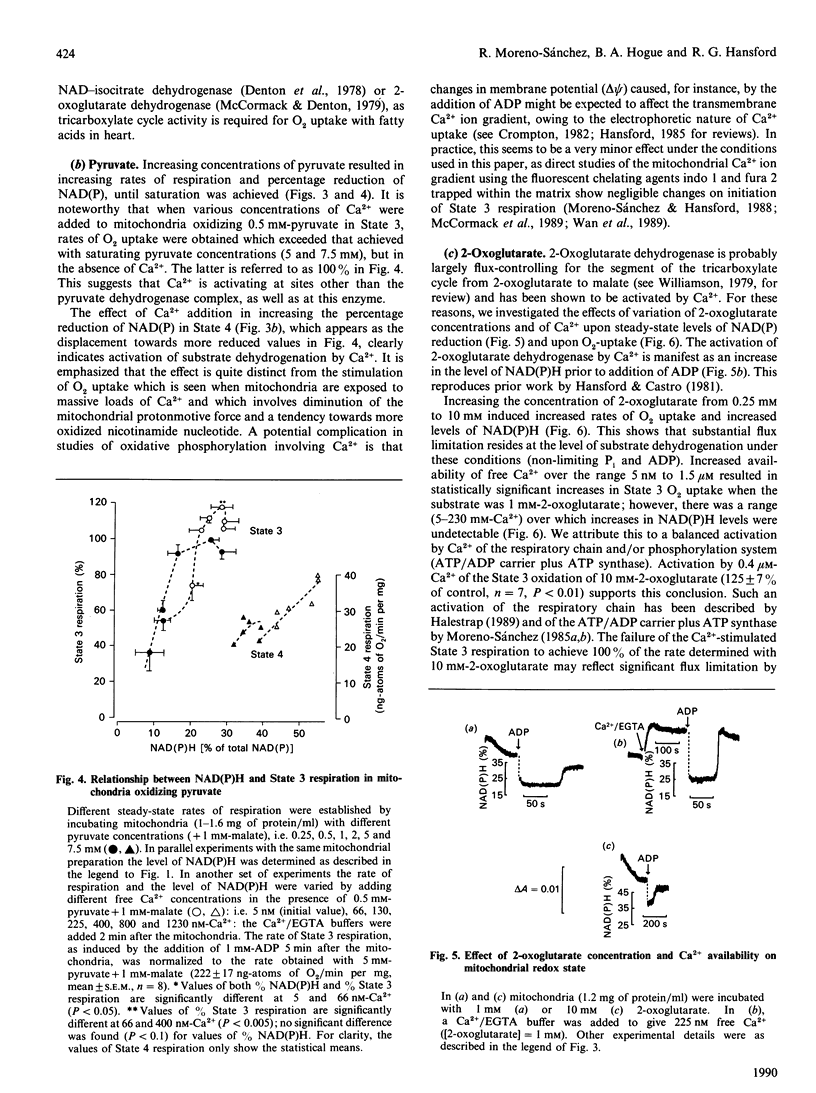
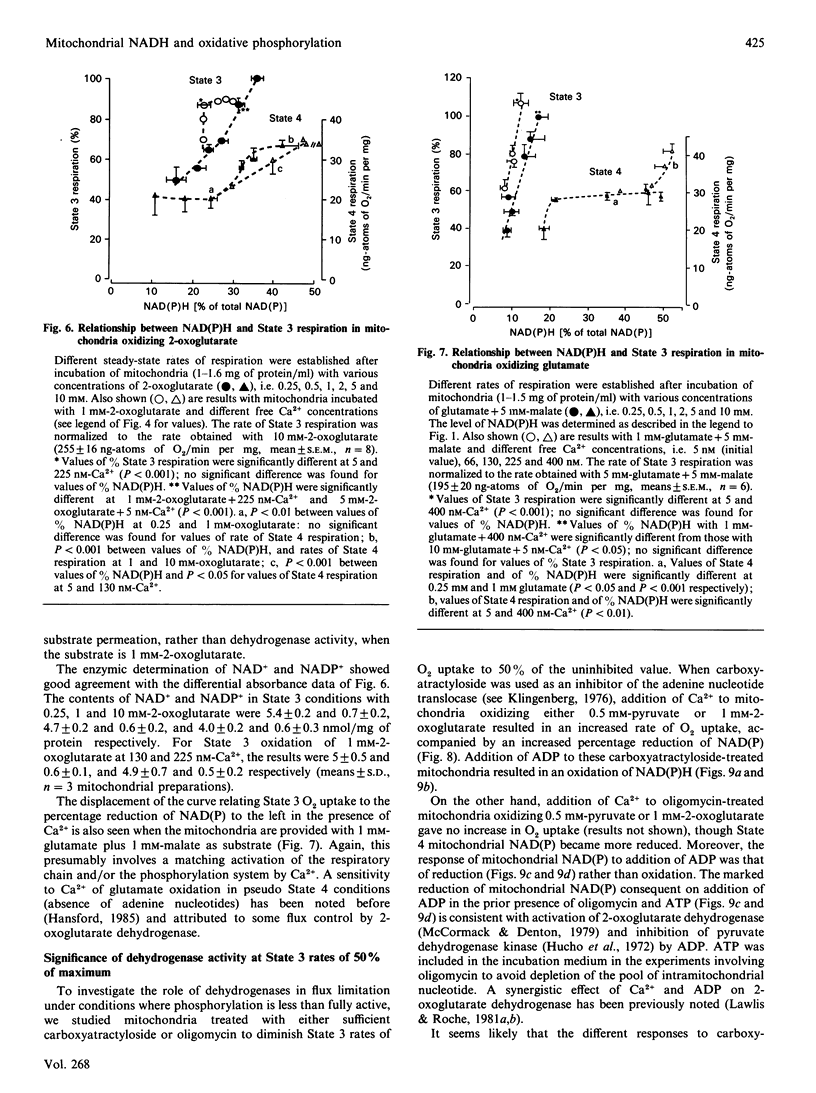
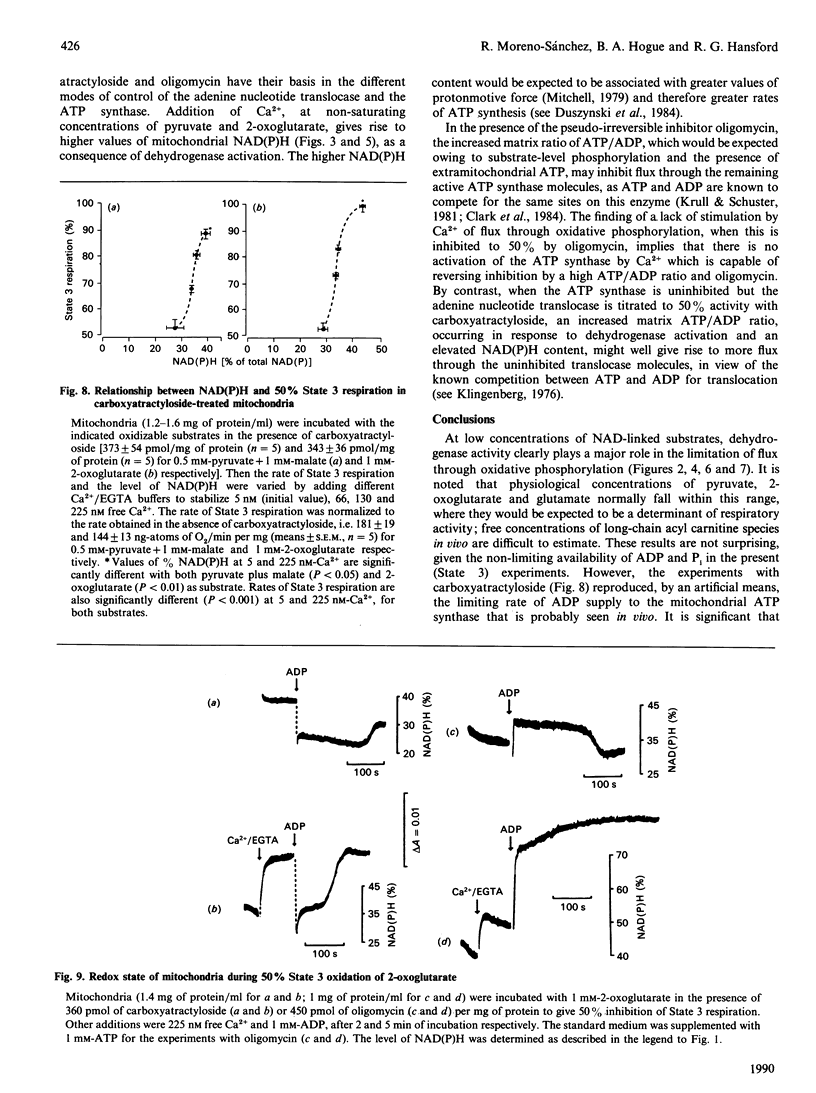
1. We have examined systematically the relationship between the percentage reduction of cardiac mitochondrial NAD and the flux through oxidative phosphorylation, as measured by O2 uptake. Reduction of NAD was varied by varying the concentration of palmitoyl-L-carnitine, pyruvate, 2-oxoglutarate or glutamate in the presence of malate as the oxidizable substrate. 2. In the presence of ADP (State 3 respiration) there was a substantially linear positive relationship between O2 uptake and the percentage reduction of NAD. Coupled respiration in the absence of ADP also showed an increase with increasing NADH, with the exact shape of the relationship being variable. 3. When pyruvate and 2-oxoglutarate dehydrogenase activity were increased by increasing medium Ca2+ concentration within the range 5 nM to 1.23 microM, at non-saturating substrate concentrations, there was again a positive relationship between O2 uptake and the reduction of NAD; however, rates of O2 uptake tended to be higher at given values of NAD reduction when the incubation medium contained Ca2+. This is taken to indicate an activation by Ca2+ of the enzymes of phosphorylation or of the respiratory chain, in addition to the dehydrogenase activation. 4. When carboxyatractyloside plus ADP were used to generate 50% State 3 rates of O2 uptake with pyruvate or 2-oxoglutarate, sensitivity to Ca2+ was retained. However, when oligomycin plus 1 mM-ADP and 1 mM-ATP were used to generate 50% State 3, no such dependence was seen. 5. The results are interpreted to indicate a substantial role for substrate dehydrogenation in the overall regulation of oxidative phosphorylation when substrates are available at near-physiological concentrations.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assimacopoulos-Jeannet F., McCormack J. G., Jeanrenaud B. Effect of phenylephrine on pyruvate dehydrogenase activity in rat hepatocytes and its interaction with insulin and glucagon. FEBS Lett. 1983 Aug 8;159(1-2):83–88. doi: 10.1016/0014-5793(83)80421-9. [DOI] [PubMed] [Google Scholar]
- Azzone G. F., Pozzan T., Massari S., Bragadin M. Proton electrochemical gradient and rate of controlled respiration in mitochondria. Biochim Biophys Acta. 1978 Feb 9;501(2):296–306. doi: 10.1016/0005-2728(78)90035-x. [DOI] [PubMed] [Google Scholar]
- Baggetto L., Gautheron D. C., Godinot C. Effects of ATP on various steps controlling the rate of oxidative phosphorylation in newborn rat liver mitochondria. Arch Biochem Biophys. 1984 Aug 1;232(2):670–678. doi: 10.1016/0003-9861(84)90587-3. [DOI] [PubMed] [Google Scholar]
- Balaban R. S., Blum J. J. Hormone-induced changes in NADH fluorescence and O2 consumption of rat hepatocytes. Am J Physiol. 1982 Mar;242(3):C172–C177. doi: 10.1152/ajpcell.1982.242.3.C172. [DOI] [PubMed] [Google Scholar]
- Balaban R. S., Kantor H. L., Katz L. A., Briggs R. W. Relation between work and phosphate metabolite in the in vivo paced mammalian heart. Science. 1986 May 30;232(4754):1121–1123. doi: 10.1126/science.3704638. [DOI] [PubMed] [Google Scholar]
- Bohnensack R., Küster U., Letko G. Rate-controlling steps of oxidative phosphorylation in rat liver mitochondria. A synoptic approach of model and experiment. Biochim Biophys Acta. 1982 Jun 18;680(3):271–280. doi: 10.1016/0005-2728(82)90139-6. [DOI] [PubMed] [Google Scholar]
- Brand M. D., Hafner R. P., Brown G. C. Control of respiration in non-phosphorylating mitochondria is shared between the proton leak and the respiratory chain. Biochem J. 1988 Oct 15;255(2):535–539. [PMC free article] [PubMed] [Google Scholar]
- Bünger R., Permanetter B. Parallel stimulation by Ca2+ of inotropism and pyruvate dehydrogenase in perfused heart. Am J Physiol. 1984 Jul;247(1 Pt 1):C45–C52. doi: 10.1152/ajpcell.1984.247.1.C45. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. III. The steady state. J Biol Chem. 1955 Nov;217(1):409–427. [PubMed] [Google Scholar]
- Charest R., Blackmore P. F., Berthon B., Exton J. H. Changes in free cytosolic Ca2+ in hepatocytes following alpha 1-adrenergic stimulation. Studies on Quin-2-loaded hepatocytes. J Biol Chem. 1983 Jul 25;258(14):8769–8773. [PubMed] [Google Scholar]
- Clark D. D., Daggett S. G., Schuster S. M. Pre-steady-state kinetics of beef heart mitochondrial ATPase. Arch Biochem Biophys. 1984 Sep;233(2):378–392. doi: 10.1016/0003-9861(84)90459-4. [DOI] [PubMed] [Google Scholar]
- Davis E. J., Davis-Van Thienen W. I. Rate control of phosphorylation-coupled respiration by rat liver mitochondria. Arch Biochem Biophys. 1984 Sep;233(2):573–581. doi: 10.1016/0003-9861(84)90481-8. [DOI] [PubMed] [Google Scholar]
- De Gómez-Puyou M. T., Gavilanes M., Gómez-Puyou A., Ernster L. Control of activity states of heart mitochondrial ATPase. Role of the proton-motive force and Ca2+. Biochim Biophys Acta. 1980 Oct 3;592(3):396–405. doi: 10.1016/0005-2728(80)90087-0. [DOI] [PubMed] [Google Scholar]
- Denton R. M., McCormack J. G. Ca2+ transport by mammalian mitochondria and its role in hormone action. Am J Physiol. 1985 Dec;249(6 Pt 1):E543–E554. doi: 10.1152/ajpendo.1985.249.6.E543. [DOI] [PubMed] [Google Scholar]
- Denton R. M., Richards D. A., Chin J. G. Calcium ions and the regulation of NAD+-linked isocitrate dehydrogenase from the mitochondria of rat heart and other tissues. Biochem J. 1978 Dec 15;176(3):899–906. doi: 10.1042/bj1760899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doussiere J., Ligeti E., Brandolin G., Vignais P. V. Control of oxidative phosphorylation in rat heart mitochondria. The role of the adenine nucleotide carrier. Biochim Biophys Acta. 1984 Aug 31;766(2):492–500. doi: 10.1016/0005-2728(84)90265-2. [DOI] [PubMed] [Google Scholar]
- Duszyński J., Bogucka K., Wojtczak L. Homeostasis of the protonmotive force in phosphorylating mitochondria. Biochim Biophys Acta. 1984 Dec 18;767(3):540–547. doi: 10.1016/0005-2728(84)90053-7. [DOI] [PubMed] [Google Scholar]
- From A. H., Petein M. A., Michurski S. P., Zimmer S. D., Uğurbil K. 31P-NMR studies of respiratory regulation in the intact myocardium. FEBS Lett. 1986 Oct 6;206(2):257–261. doi: 10.1016/0014-5793(86)80992-9. [DOI] [PubMed] [Google Scholar]
- Groen A. K., Wanders R. J., Westerhoff H. V., van der Meer R., Tager J. M. Quantification of the contribution of various steps to the control of mitochondrial respiration. J Biol Chem. 1982 Mar 25;257(6):2754–2757. [PubMed] [Google Scholar]
- Gómez-Puyou A., Ayala G., Muller U., Tuena de Gómez-Puyou M. Regulation of the synthesis and hydrolysis of ATP by mitochondrial ATPase. Role of Mg2+. J Biol Chem. 1983 Nov 25;258(22):13673–13679. [PubMed] [Google Scholar]
- Halestrap A. P. The regulation of the matrix volume of mammalian mitochondria in vivo and in vitro and its role in the control of mitochondrial metabolism. Biochim Biophys Acta. 1989 Mar 23;973(3):355–382. doi: 10.1016/s0005-2728(89)80378-0. [DOI] [PubMed] [Google Scholar]
- Halestrap A. P. The regulation of the oxidation of fatty acids and other substrates in rat heart mitochondria by changes in the matrix volume induced by osmotic strength, valinomycin and Ca2+. Biochem J. 1987 May 15;244(1):159–164. doi: 10.1042/bj2440159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansford R. G., Castro F. Effects of micromolar concentrations of free calcium ions on the reduction of heart mitochondrial NAD(P) by 2-oxoglutarate. Biochem J. 1981 Sep 15;198(3):525–533. doi: 10.1042/bj1980525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansford R. G., Johnson R. N. The steady state concentrations of coenzyme A-SH and coenzyme A thioester, citrate, and isocitrate during tricarboxylate cycle oxidations in rabbit heart mitochondria. J Biol Chem. 1975 Nov 10;250(21):8361–8375. [PubMed] [Google Scholar]
- Hansford R. G. Relation between mitochondrial calcium transport and control of energy metabolism. Rev Physiol Biochem Pharmacol. 1985;102:1–72. doi: 10.1007/BFb0034084. [DOI] [PubMed] [Google Scholar]
- Heinrich R., Rapoport T. A. A linear steady-state treatment of enzymatic chains. General properties, control and effector strength. Eur J Biochem. 1974 Feb 15;42(1):89–95. doi: 10.1111/j.1432-1033.1974.tb03318.x. [DOI] [PubMed] [Google Scholar]
- Hucho F., Randall D. D., Roche T. E., Burgett M. W., Pelley J. W., Reed L. J. -Keto acid dehydrogenase complexes. XVII. Kinetic and regulatory properties of pyruvate dehydrogenase kinase and pyruvate dehydrogenase phosphatase from bovine kidney and heart. Arch Biochem Biophys. 1972 Jul;151(1):328–340. doi: 10.1016/0003-9861(72)90504-8. [DOI] [PubMed] [Google Scholar]
- Kacser H., Burns J. A. The control of flux. Symp Soc Exp Biol. 1973;27:65–104. [PubMed] [Google Scholar]
- Kantor H. L., Briggs R. W., Metz K. R., Balaban R. S. Gated in vivo examination of cardiac metabolites with 31P nuclear magnetic resonance. Am J Physiol. 1986 Jul;251(1 Pt 2):H171–H175. doi: 10.1152/ajpheart.1986.251.1.H171. [DOI] [PubMed] [Google Scholar]
- Katz L. A., Koretsky A. P., Balaban R. S. Activation of dehydrogenase activity and cardiac respiration: a 31P-NMR study. Am J Physiol. 1988 Jul;255(1 Pt 2):H185–H188. doi: 10.1152/ajpheart.1988.255.1.H185. [DOI] [PubMed] [Google Scholar]
- Katz L. A., Koretsky A. P., Balaban R. S. Respiratory control in the glucose perfused heart. A 31P NMR and NADH fluorescence study. FEBS Lett. 1987 Sep 14;221(2):270–276. doi: 10.1016/0014-5793(87)80939-0. [DOI] [PubMed] [Google Scholar]
- Katz L. A., Swain J. A., Portman M. A., Balaban R. S. Relation between phosphate metabolites and oxygen consumption of heart in vivo. Am J Physiol. 1989 Jan;256(1 Pt 2):H265–H274. doi: 10.1152/ajpheart.1989.256.1.H265. [DOI] [PubMed] [Google Scholar]
- Khym J. X. An analytical system for rapid separation of tissue nucleotides at low pressures on conventional anion exchangers. Clin Chem. 1975 Aug;21(9):1245–1252. [PubMed] [Google Scholar]
- Koretsky A. P., Balaban R. S. Changes in pyridine nucleotide levels alter oxygen consumption and extra-mitochondrial phosphates in isolated mitochondria: a 31P-NMR and NAD(P)H fluorescence study. Biochim Biophys Acta. 1987 Oct 7;893(3):398–408. doi: 10.1016/0005-2728(87)90092-2. [DOI] [PubMed] [Google Scholar]
- Krull K. W., Schuster S. M. Kinetic studies of beef heart mitochondrial adenosine triphosphatase: interaction of the inhibitor protein and adenosine triphosphate analogues. Biochemistry. 1981 Mar 17;20(6):1592–1598. doi: 10.1021/bi00509a028. [DOI] [PubMed] [Google Scholar]
- Krämer R., Mayr U., Heberger C., Tsompanidou S. Activation of the ADP/ATP carrier from mitochondria by cationic effectors. Biochim Biophys Acta. 1986 Feb 27;855(2):201–210. doi: 10.1016/0005-2736(86)90166-5. [DOI] [PubMed] [Google Scholar]
- Kurlandsky S. B., Hilburger A. C., Levy H. R. Glucose-6-phosphate dehydrogenase from Leuconostoc mesenteroides: ligand-induced conformational changes. Arch Biochem Biophys. 1988 Jul;264(1):93–102. doi: 10.1016/0003-9861(88)90574-7. [DOI] [PubMed] [Google Scholar]
- LaNoue K., Nicklas W. J., Williamson J. R. Control of citric acid cycle activity in rat heart mitochondria. J Biol Chem. 1970 Jan 10;245(1):102–111. [PubMed] [Google Scholar]
- Lawlis V. B., Roche T. E. Inhibition of bovine kidney alpha-ketoglutarate dehydrogenase complex by reduced nicotinamide adenine dinucleotide in the presence or absence of calcium ion and effect of adenosine 5'-diphosphate on reduced nicotinamide adenine dinucleotide inhibition. Biochemistry. 1981 Apr 28;20(9):2519–2524. doi: 10.1021/bi00512a024. [DOI] [PubMed] [Google Scholar]
- Lawlis V. B., Roche T. E. Regulation of bovine kidney alpha-ketoglutarate dehydrogenase complex by calcium ion and adenine nucleotides. Effects on S0.5 for alpha-ketoglutarate. Biochemistry. 1981 Apr 28;20(9):2512–2518. doi: 10.1021/bi00512a023. [DOI] [PubMed] [Google Scholar]
- Matsuno-Yagi A., Hatefi Y. Kinetic modalities of ATP synthesis. Regulation by the mitochondrial respiratory chain. J Biol Chem. 1986 Oct 25;261(30):14031–14038. [PubMed] [Google Scholar]
- McCormack J. G., Browne H. M., Dawes N. J. Studies on mitochondrial Ca2+-transport and matrix Ca2+ using fura-2-loaded rat heart mitochondria. Biochim Biophys Acta. 1989 Mar 23;973(3):420–427. doi: 10.1016/s0005-2728(89)80384-6. [DOI] [PubMed] [Google Scholar]
- McCormack J. G., Denton R. M. The effects of calcium ions and adenine nucleotides on the activity of pig heart 2-oxoglutarate dehydrogenase complex. Biochem J. 1979 Jun 15;180(3):533–544. doi: 10.1042/bj1800533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P. The Ninth Sir Hans Krebs Lecture. Compartmentation and communication in living systems. Ligand conduction: a general catalytic principle in chemical, osmotic and chemiosmotic reaction systems. Eur J Biochem. 1979 Mar 15;95(1):1–20. doi: 10.1111/j.1432-1033.1979.tb12934.x. [DOI] [PubMed] [Google Scholar]
- Moreno-Sánchez R. Contribution of the translocator of adenine nucleotides and the ATP synthase to the control of oxidative phosphorylation and arsenylation in liver mitochondria. J Biol Chem. 1985 Oct 15;260(23):12554–12560. [PubMed] [Google Scholar]
- Moreno-Sánchez R., Hansford R. G. Dependence of cardiac mitochondrial pyruvate dehydrogenase activity on intramitochondrial free Ca2+ concentration. Biochem J. 1988 Dec 1;256(2):403–412. doi: 10.1042/bj2560403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Sánchez R. Regulation of oxidative phosphorylation in mitochondria by external free Ca2+ concentrations. J Biol Chem. 1985 Apr 10;260(7):4028–4034. [PubMed] [Google Scholar]
- Nicholls D. G. The influence of respiration and ATP hydrolysis on the proton-electrochemical gradient across the inner membrane of rat-liver mitochondria as determined by ion distribution. Eur J Biochem. 1974 Dec 16;50(1):305–315. doi: 10.1111/j.1432-1033.1974.tb03899.x. [DOI] [PubMed] [Google Scholar]
- Staddon J. M., Hansford R. G. 4 beta-Phorbol 12-myristate 13-acetate attenuates the glucagon-induced increase in cytoplasmic free Ca2+ concentration in isolated rat hepatocytes. Biochem J. 1986 Sep 15;238(3):737–743. doi: 10.1042/bj2380737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staddon J. M., Hansford R. G. The glucagon-induced activation of pyruvate dehydrogenase in hepatocytes is diminished by 4 beta-phorbol 12-myristate 13-acetate. A role for cytoplasmic Ca2+ in dehydrogenase regulation. Biochem J. 1987 Feb 1;241(3):729–735. doi: 10.1042/bj2410729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden M. C., Ball A. J., Williamson D. H. A second site of vasopressin action on [1-14C]oleate metabolism in isolated rat hepatocytes: increased formation of 14CO2. Biochem Soc Trans. 1980 Oct;8(5):591–592. doi: 10.1042/bst0080591. [DOI] [PubMed] [Google Scholar]
- Vinogradov A., Scarpa A., Chance B. Calcium and pyridine nucleotide interaction in mitochondrial membranes. Arch Biochem Biophys. 1972 Oct;152(2):646–654. doi: 10.1016/0003-9861(72)90261-5. [DOI] [PubMed] [Google Scholar]
- Wan B., LaNoue K. F., Cheung J. Y., Scaduto R. C., Jr Regulation of citric acid cycle by calcium. J Biol Chem. 1989 Aug 15;264(23):13430–13439. [PubMed] [Google Scholar]
- Wiesner R. J., Kreutzer U., Rösen P., Grieshaber M. K. Subcellular distribution of malate-aspartate cycle intermediates during normoxia and anoxia in the heart. Biochim Biophys Acta. 1988 Oct 26;936(1):114–123. doi: 10.1016/0005-2728(88)90258-7. [DOI] [PubMed] [Google Scholar]
- Williamson J. R. Mitochondrial function in the heart. Annu Rev Physiol. 1979;41:485–506. doi: 10.1146/annurev.ph.41.030179.002413. [DOI] [PubMed] [Google Scholar]
- Wilson D. F., Owen C. S., Holian A. Control of mitochondrial respiration: a quantitative evaluation of the roles of cytochrome c and oxygen. Arch Biochem Biophys. 1977 Aug;182(2):749–762. doi: 10.1016/0003-9861(77)90557-4. [DOI] [PubMed] [Google Scholar]
- Woelders H., van der Velden T., van Dam K. Unique relationships between the rates of oxidation and phosphorylation and the protonmotive force in rat-liver mitochondria. Biochim Biophys Acta. 1988 Jun 15;934(1):123–134. doi: 10.1016/0005-2728(88)90127-2. [DOI] [PubMed] [Google Scholar]
- Yamada E. W., Huzel N. J. Isolation of two ATPase inhibitor proteins from mitochondria of rat skeletal muscle. Biosci Rep. 1983 Oct;3(10):947–954. doi: 10.1007/BF01140664. [DOI] [PubMed] [Google Scholar]
- Yamada E. W., Huzel N. J. The calcium-binding ATPase inhibitor protein from bovine heart mitochondria. Purification and properties. J Biol Chem. 1988 Aug 15;263(23):11498–11503. [PubMed] [Google Scholar]
- van der Meer R., Akerboom T. P., Groen A. K., Tager J. M. Relationship between oxygen uptake of perifused rat-liver cells and the cytosolic phosphorylation state calculated from indicator metabolites and a redetermined equilibrium constant. Eur J Biochem. 1978 Mar 15;84(2):421–428. doi: 10.1111/j.1432-1033.1978.tb12183.x. [DOI] [PubMed] [Google Scholar]


