Abstract
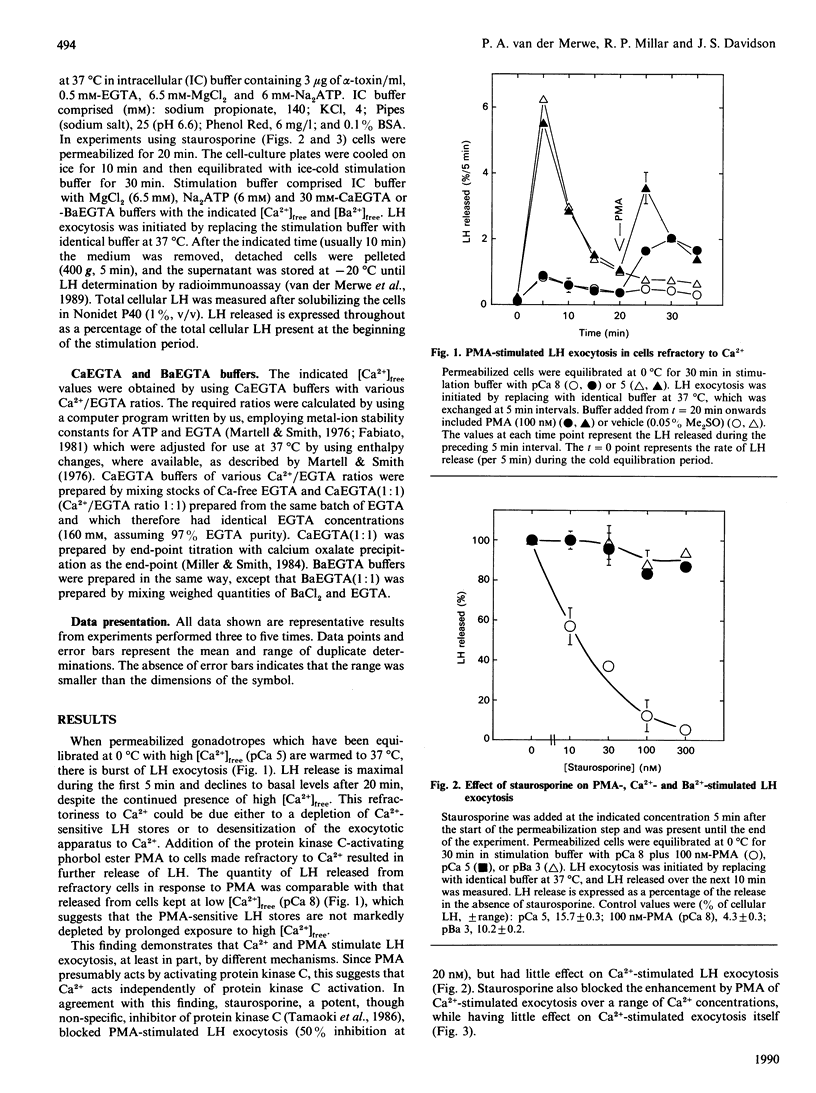
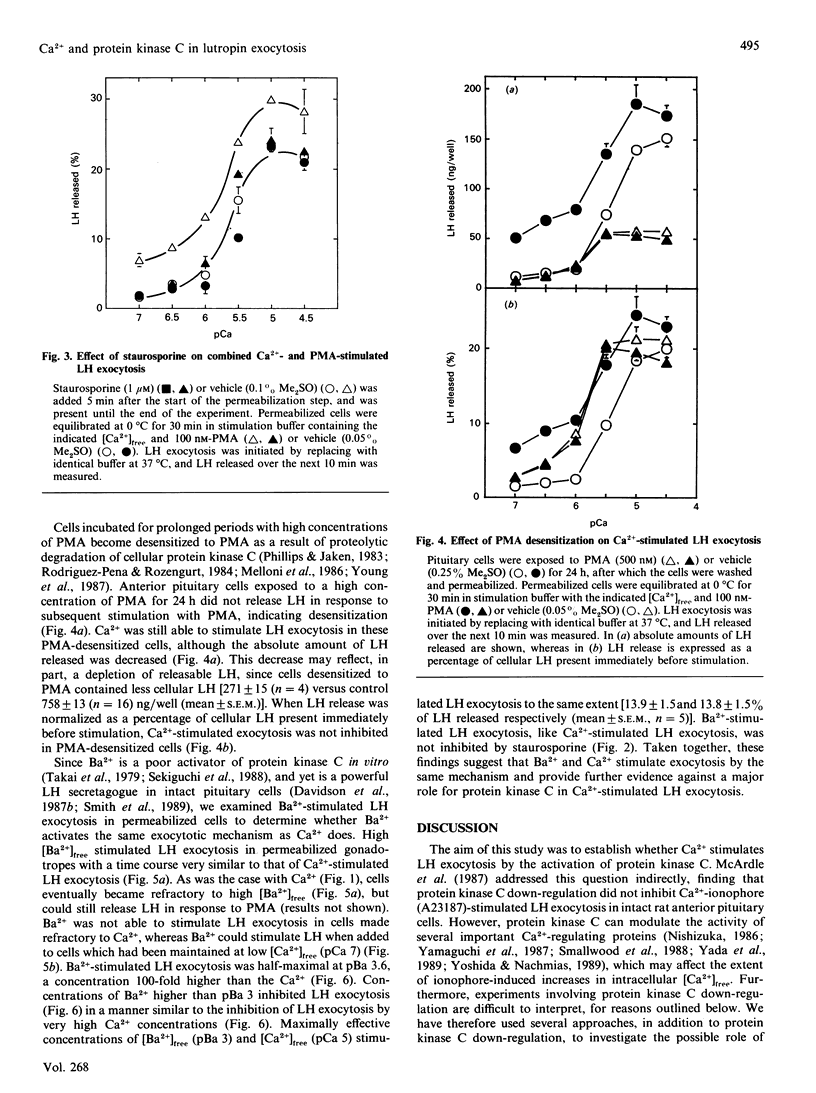
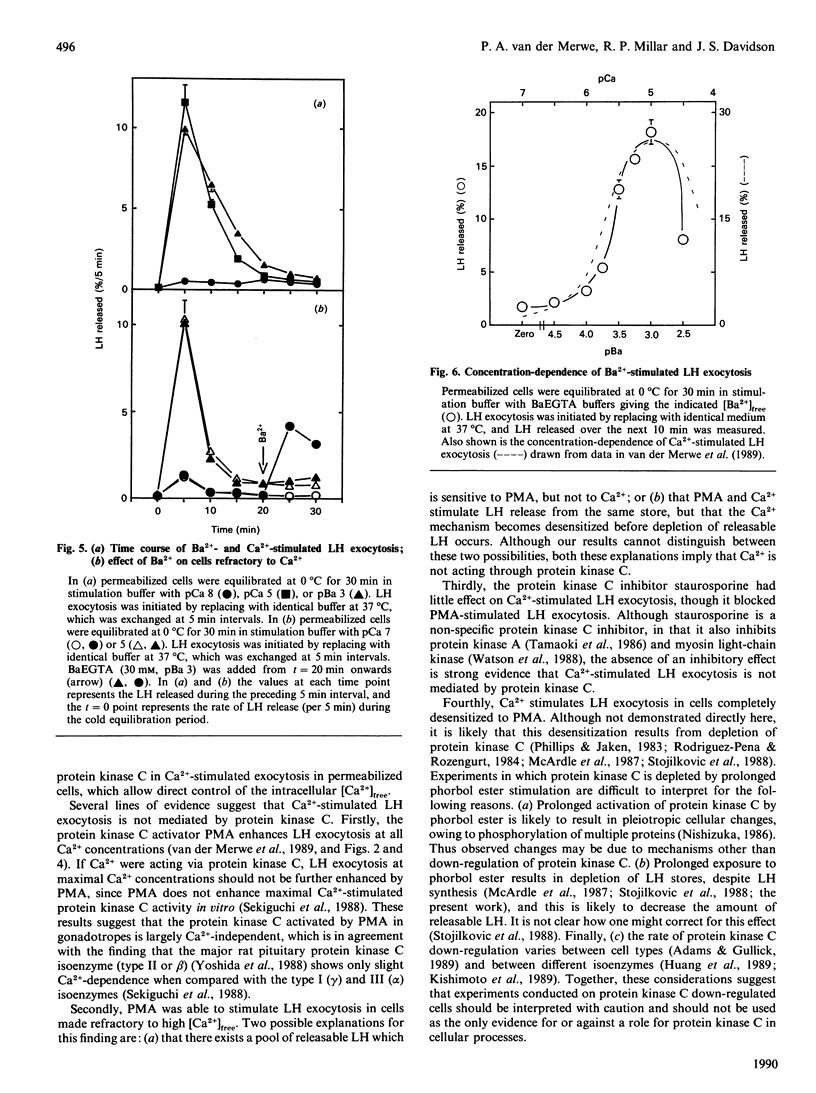
Using permeabilized gonadotropes, we examined whether Ca2(+)-stimulated luteinizing-hormone (LH) exocytosis is mediated by the Ca2(+)-activated phospholipid-dependent protein kinase (protein kinase C). In the presence of high [Ca2+]free (pCa 5), alpha-toxin-permeabilized sheep gonadotropes secrete a burst of LH and then become refractory to maintained high [Ca2+]free. The protein kinase C activator phorbol myristate acetate (PMA) is able to stimulate further LH release from cells made refractory to high [Ca2+]free, suggesting that Ca2+ does not stimulate LH release by activating protein kinase C. Staurosporine, a protein kinase C inhibitor, inhibited PMA-stimulated (50% inhibition at 20 nM), but not Ca2(+)-stimulated, LH exocytosis. In cells desensitized to PMA by prolonged exposure to a high PMA concentration, Ca2(+)-stimulated LH exocytosis (when corrected for depletion of total cellular LH) was not inhibited. Ba2+ was able to stimulate LH exocytosis to a maximal extent similar to Ca2+, although higher Ba2+ concentrations were necessary. Ba2+ and Ca2+ stimulated LH exocytosis with a similar time course, and both were inhibitory at high concentrations. Furthermore, cells made refractory to Ca2+ were also refractory to Ba2+. These data strongly suggest that Ba2+ and Ca2+ act through the same mechanism. Since Ba2+ is a poor activator of protein kinase C, these findings are additional evidence against a major role for protein kinase C in mediating Ca2(+)-stimulated LH exocytosis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. C., Gullick W. J. Differences in phorbol-ester-induced down-regulation of protein kinase C between cell lines. Biochem J. 1989 Feb 1;257(3):905–911. doi: 10.1042/bj2570905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Knight D. E. Exocytosis: control by calcium and other factors. Br Med Bull. 1986 Oct;42(4):399–404. doi: 10.1093/oxfordjournals.bmb.a072158. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Geisow M. J. The annexin family of calcium-binding proteins. Review article. Cell Calcium. 1989 Jan;10(1):1–10. doi: 10.1016/0143-4160(89)90038-9. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Morgan A., O'Sullivan A. J. A major role for protein kinase C in calcium-activated exocytosis in permeabilised adrenal chromaffin cells. FEBS Lett. 1988 Sep 26;238(1):151–155. doi: 10.1016/0014-5793(88)80246-1. [DOI] [PubMed] [Google Scholar]
- Chao S. H., Suzuki Y., Zysk J. R., Cheung W. Y. Activation of calmodulin by various metal cations as a function of ionic radius. Mol Pharmacol. 1984 Jul;26(1):75–82. [PubMed] [Google Scholar]
- Conn P. M. Does protein kinase C mediate pituitary actions of gonadotropin-releasing hormone? Mol Endocrinol. 1989 May;3(5):755–757. doi: 10.1210/mend-3-5-755. [DOI] [PubMed] [Google Scholar]
- Conn P. M., McArdle C. A., Andrews W. V., Huckle W. R. The molecular basis of gonadotropin-releasing hormone (GnRH) action in the pituitary gonadotrope. Biol Reprod. 1987 Feb;36(1):17–35. doi: 10.1095/biolreprod36.1.17. [DOI] [PubMed] [Google Scholar]
- Conn P. M., Rogers D. C., Sheffield T. Inhibition of gonadotropin-releasing hormone-stimulated luteinizing hormone release by pimozide: evidence for a site of action after calcium mobilization. Endocrinology. 1981 Oct;109(4):1122–1126. doi: 10.1210/endo-109-4-1122. [DOI] [PubMed] [Google Scholar]
- Davidson J. S., King J. A., Millar R. P. Luteinizing hormone release from chicken pituitary cells: synergism between calcium and protein kinase C and its inhibition by calmodulin antagonists. Endocrinology. 1987 Feb;120(2):692–699. doi: 10.1210/endo-120-2-692. [DOI] [PubMed] [Google Scholar]
- Davidson J. S., Wakefield I., King J. A., Millar R. P. Barium-induced LH release from chicken pituitary cells: synergism with phorbol ester. J Endocrinol. 1987 Jul;114(1):11–16. doi: 10.1677/joe.0.1140011. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Myoplasmic free calcium concentration reached during the twitch of an intact isolated cardiac cell and during calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned cardiac cell from the adult rat or rabbit ventricle. J Gen Physiol. 1981 Nov;78(5):457–497. doi: 10.1085/jgp.78.5.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietzen K., Wüthrich A., Bader H. R 24571: a new powerful inhibitor of red blood cell Ca++-transport ATPase and of calmodulin-regulated functions. Biochem Biophys Res Commun. 1981 Jul 30;101(2):418–425. doi: 10.1016/0006-291x(81)91276-6. [DOI] [PubMed] [Google Scholar]
- Hii C. S., Jones P. M., Persaud S. J., Howell S. L. A re-assessment of the role of protein kinase C in glucose-stimulated insulin secretion. Biochem J. 1987 Sep 1;246(2):489–493. doi: 10.1042/bj2460489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F. L., Yoshida Y., Cunha-Melo J. R., Beaven M. A., Huang K. P. Differential down-regulation of protein kinase C isozymes. J Biol Chem. 1989 Mar 5;264(7):4238–4243. [PubMed] [Google Scholar]
- Huckle W. R., Conn P. M. Molecular mechanism of gonadotropin releasing hormone action. II. The effector system. Endocr Rev. 1988 Nov;9(4):387–395. doi: 10.1210/edrv-9-4-387. [DOI] [PubMed] [Google Scholar]
- Kishimoto A., Mikawa K., Hashimoto K., Yasuda I., Tanaka S., Tominaga M., Kuroda T., Nishizuka Y. Limited proteolysis of protein kinase C subspecies by calcium-dependent neutral protease (calpain). J Biol Chem. 1989 Mar 5;264(7):4088–4092. [PubMed] [Google Scholar]
- Knight D. E., Sugden D., Baker P. F. Evidence implicating protein kinase C in exocytosis from electropermeabilized bovine chromaffin cells. J Membr Biol. 1988 Aug;104(1):21–34. doi: 10.1007/BF01871899. [DOI] [PubMed] [Google Scholar]
- Kuret J., Schulman H. Purification and characterization of a Ca2+/calmodulin-dependent protein kinase from rat brain. Biochemistry. 1984 Nov 6;23(23):5495–5504. doi: 10.1021/bi00318a018. [DOI] [PubMed] [Google Scholar]
- Limor R., Ayalon D., Capponi A. M., Childs G. V., Naor Z. Cytosolic free calcium levels in cultured pituitary cells separated by centrifugal elutriation: effect of gonadotropin-releasing hormone. Endocrinology. 1987 Feb;120(2):497–503. doi: 10.1210/endo-120-2-497. [DOI] [PubMed] [Google Scholar]
- Martin T. F., Walent J. H. A new method for cell permeabilization reveals a cytosolic protein requirement for Ca2+ -activated secretion in GH3 pituitary cells. J Biol Chem. 1989 Jun 15;264(17):10299–10308. [PubMed] [Google Scholar]
- Martin W. H., Creutz C. E. Chromobindin A. A Ca2+ and ATP regulated chromaffin granule binding protein. J Biol Chem. 1987 Feb 25;262(6):2803–2810. [PubMed] [Google Scholar]
- Matthies H. J., Palfrey H. C., Miller R. J. Calmodulin- and protein phosphorylation-independent release of catecholamines from PC-12 cells. FEBS Lett. 1988 Mar 14;229(2):238–242. doi: 10.1016/0014-5793(88)81132-3. [DOI] [PubMed] [Google Scholar]
- McArdle C. A., Huckle W. R., Conn P. M. Phorbol esters reduce gonadotrope responsiveness to protein kinase C activators but not to Ca2+-mobilizing secretagogues. Does protein kinase C mediate gonadotropin-releasing hormone action? J Biol Chem. 1987 Apr 15;262(11):5028–5035. [PubMed] [Google Scholar]
- McLaughlin S., Whitaker M. Cations that alter surface potentials of lipid bilayers increase the calcium requirement for exocytosis in sea urchin eggs. J Physiol. 1988 Feb;396:189–204. doi: 10.1113/jphysiol.1988.sp016958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melloni E., Pontremoli S., Michetti M., Sacco O., Sparatore B., Horecker B. L. The involvement of calpain in the activation of protein kinase C in neutrophils stimulated by phorbol myristic acid. J Biol Chem. 1986 Mar 25;261(9):4101–4105. [PubMed] [Google Scholar]
- Miller D. J., Smith G. L. EGTA purity and the buffering of calcium ions in physiological solutions. Am J Physiol. 1984 Jan;246(1 Pt 1):C160–C166. doi: 10.1152/ajpcell.1984.246.1.C160. [DOI] [PubMed] [Google Scholar]
- Moore P. B., Dedman J. R. Calcium-dependent protein binding to phenothiazine columns. J Biol Chem. 1982 Aug 25;257(16):9663–9667. [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Phillips M. A., Jaken S. Specific desensitization to tumor-promoting phorbol esters in mouse pituitary cells. Evidence that desensitization is a two-step process. J Biol Chem. 1983 Mar 10;258(5):2875–2881. [PubMed] [Google Scholar]
- Pollard H. B., Burns A. L., Rojas E. A molecular basis for synexin-driven, calcium-dependent membrane fusion. J Exp Biol. 1988 Sep;139:267–286. doi: 10.1242/jeb.139.1.267. [DOI] [PubMed] [Google Scholar]
- Rando R. R. Regulation of protein kinase C activity by lipids. FASEB J. 1988 May;2(8):2348–2355. doi: 10.1096/fasebj.2.8.3282960. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pena A., Rozengurt E. Disappearance of Ca2+-sensitive, phospholipid-dependent protein kinase activity in phorbol ester-treated 3T3 cells. Biochem Biophys Res Commun. 1984 May 16;120(3):1053–1059. doi: 10.1016/s0006-291x(84)80213-2. [DOI] [PubMed] [Google Scholar]
- Sanchez A., Hallam T. J., Rink T. J. Trifluoperazine and chlorpromazine block secretion from human platelets evoked at basal cytoplasmic free calcium by activators of C-kinase. FEBS Lett. 1983 Nov 28;164(1):43–46. doi: 10.1016/0014-5793(83)80015-5. [DOI] [PubMed] [Google Scholar]
- Sekiguchi K., Tsukuda M., Ase K., Kikkawa U., Nishizuka Y. Mode of activation and kinetic properties of three distinct forms of protein kinase C from rat brain. J Biochem. 1988 May;103(5):759–765. doi: 10.1093/oxfordjournals.jbchem.a122343. [DOI] [PubMed] [Google Scholar]
- Shangold G. A., Murphy S. N., Miller R. J. Gonadotropin-releasing hormone-induced Ca2+ transients in single identified gonadotropes require both intracellular Ca2+ mobilization and Ca2+ influx. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6566–6570. doi: 10.1073/pnas.85.17.6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood J. I., Gügi B., Rasmussen H. Regulation of erythrocyte Ca2+ pump activity by protein kinase C. J Biol Chem. 1988 Feb 15;263(5):2195–2202. [PubMed] [Google Scholar]
- Smith C. E., Davidson J. S., Millar R. P. Ba2+ stimulation of luteinizing-hormone release demonstrates two mechanisms of Ca2+ entry in gonadotrope cells. Biochem J. 1989 Apr 1;259(1):217–221. doi: 10.1042/bj2590217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojilković S. S., Chang J. P., Ngo D., Catt K. J. Evidence for a role of protein kinase C in luteinizing hormone synthesis and secretion. Impaired responses to gonadotropin-releasing hormone in protein kinase C-depleted pituitary cells. J Biol Chem. 1988 Nov 25;263(33):17307–17311. [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Iwasa Y., Kawahara Y., Mori T., Nishizuka Y. Calcium-dependent activation of a multifunctional protein kinase by membrane phospholipids. J Biol Chem. 1979 May 25;254(10):3692–3695. [PubMed] [Google Scholar]
- Tamaoki T., Nomoto H., Takahashi I., Kato Y., Morimoto M., Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca++dependent protein kinase. Biochem Biophys Res Commun. 1986 Mar 13;135(2):397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Tasaka K., Stojilkovic S. S., Izumi S., Catt K. J. Biphasic activation of cytosolic free calcium and LH responses by gonadotropin-releasing hormone. Biochem Biophys Res Commun. 1988 Jul 15;154(1):398–403. doi: 10.1016/0006-291x(88)90699-7. [DOI] [PubMed] [Google Scholar]
- Watson S. P., McNally J., Shipman L. J., Godfrey P. P. The action of the protein kinase C inhibitor, staurosporine, on human platelets. Evidence against a regulatory role for protein kinase C in the formation of inositol trisphosphate by thrombin. Biochem J. 1988 Jan 15;249(2):345–350. doi: 10.1042/bj2490345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeds A. Actin-binding proteins--regulators of cell architecture and motility. Nature. 1982 Apr 29;296(5860):811–816. doi: 10.1038/296811a0. [DOI] [PubMed] [Google Scholar]
- Yada T., Russo L. L., Sharp G. W. Phorbol ester-stimulated insulin secretion by RINm5F insulinoma cells is linked with membrane depolarization and an increase in cytosolic free Ca2+ concentration. J Biol Chem. 1989 Feb 15;264(5):2455–2462. [PubMed] [Google Scholar]
- Yamaguchi D. T., Kleeman C. R., Muallem S. Protein kinase C-activated calcium channel in the osteoblast-like clonal osteosarcoma cell line UMR-106. J Biol Chem. 1987 Nov 5;262(31):14967–14973. [PubMed] [Google Scholar]
- Yoshida K., Nachmias V. T. Calcium sequestration in human platelets: is it stimulated by protein kinase C? Cell Calcium. 1989 Jul;10(5):299–307. doi: 10.1016/0143-4160(89)90056-0. [DOI] [PubMed] [Google Scholar]
- Yoshida Y., Huang F. L., Nakabayashi H., Huang K. P. Tissue distribution and developmental expression of protein kinase C isozymes. J Biol Chem. 1988 Jul 15;263(20):9868–9873. [PubMed] [Google Scholar]
- Young S., Parker P. J., Ullrich A., Stabel S. Down-regulation of protein kinase C is due to an increased rate of degradation. Biochem J. 1987 Jun 15;244(3):775–779. doi: 10.1042/bj2440775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Merwe P. A., Millar R. P., Wakefield I. K., Davidson J. S. Mechanisms of luteinizing-hormone exocytosis in Staphylococcus aureus-alpha-toxin-permeabilized sheep gonadotropes. Biochem J. 1989 Dec 15;264(3):901–908. doi: 10.1042/bj2640901. [DOI] [PMC free article] [PubMed] [Google Scholar]


