Abstract
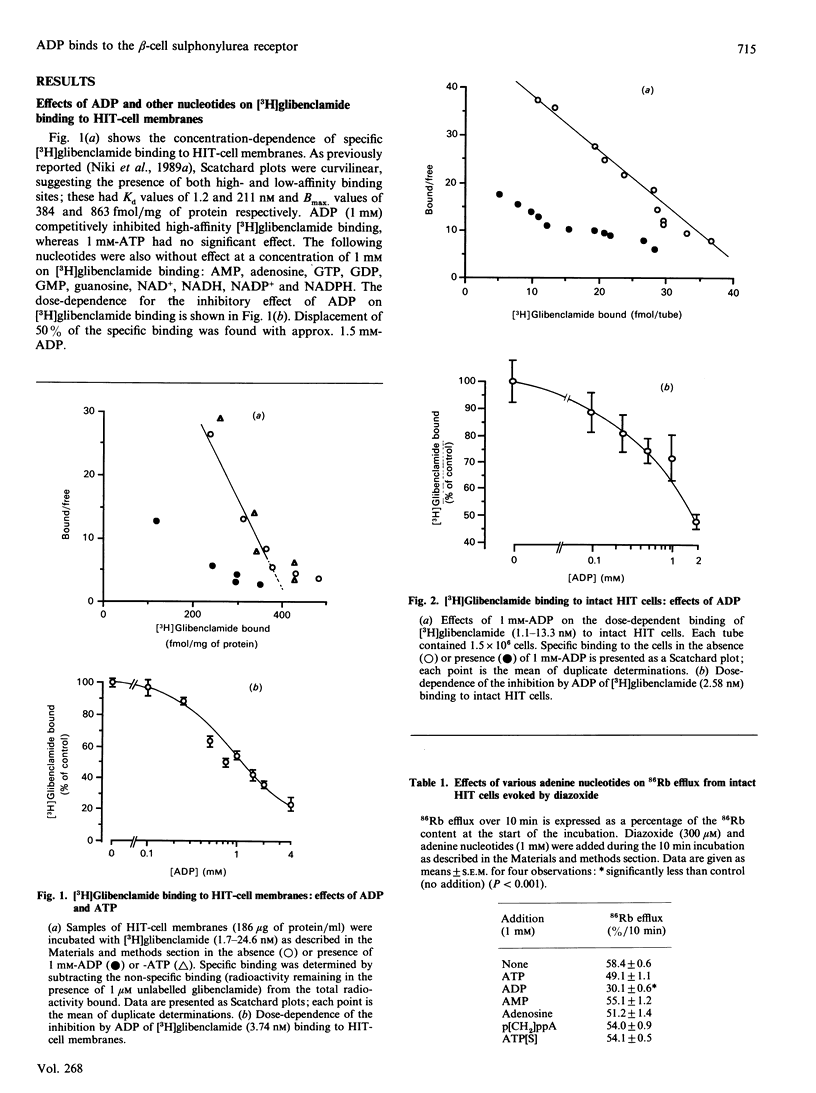
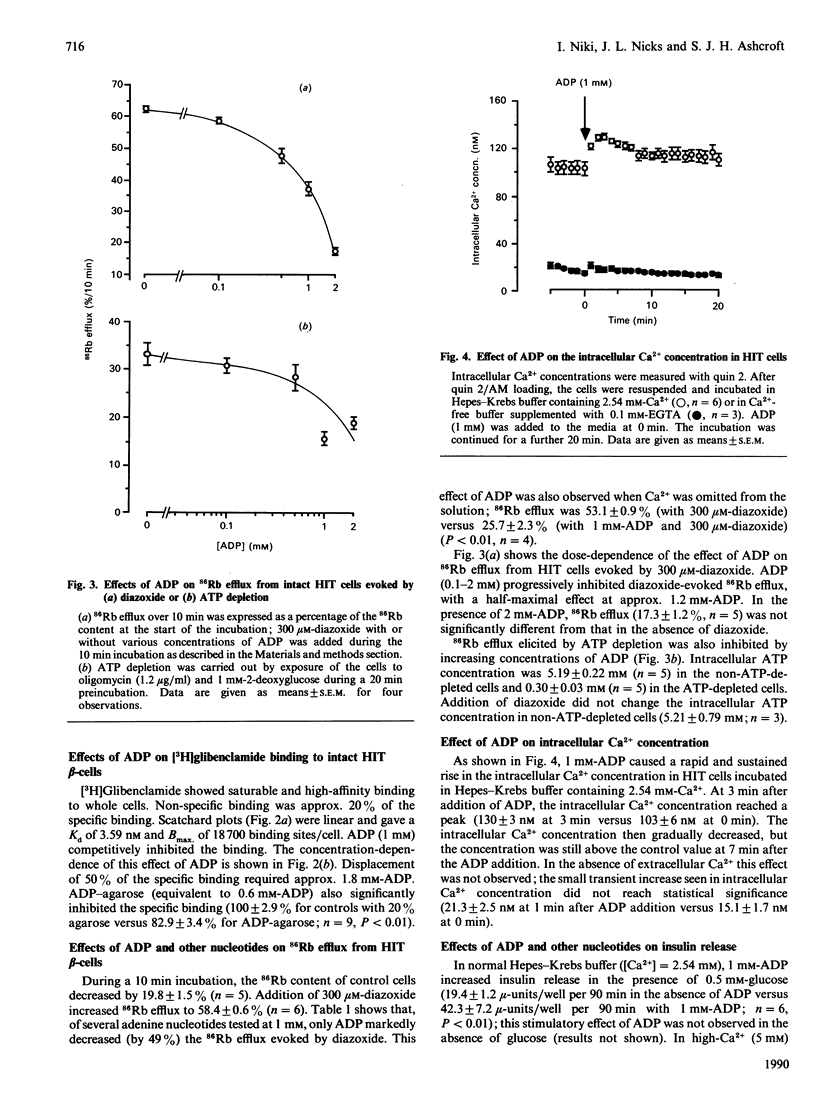
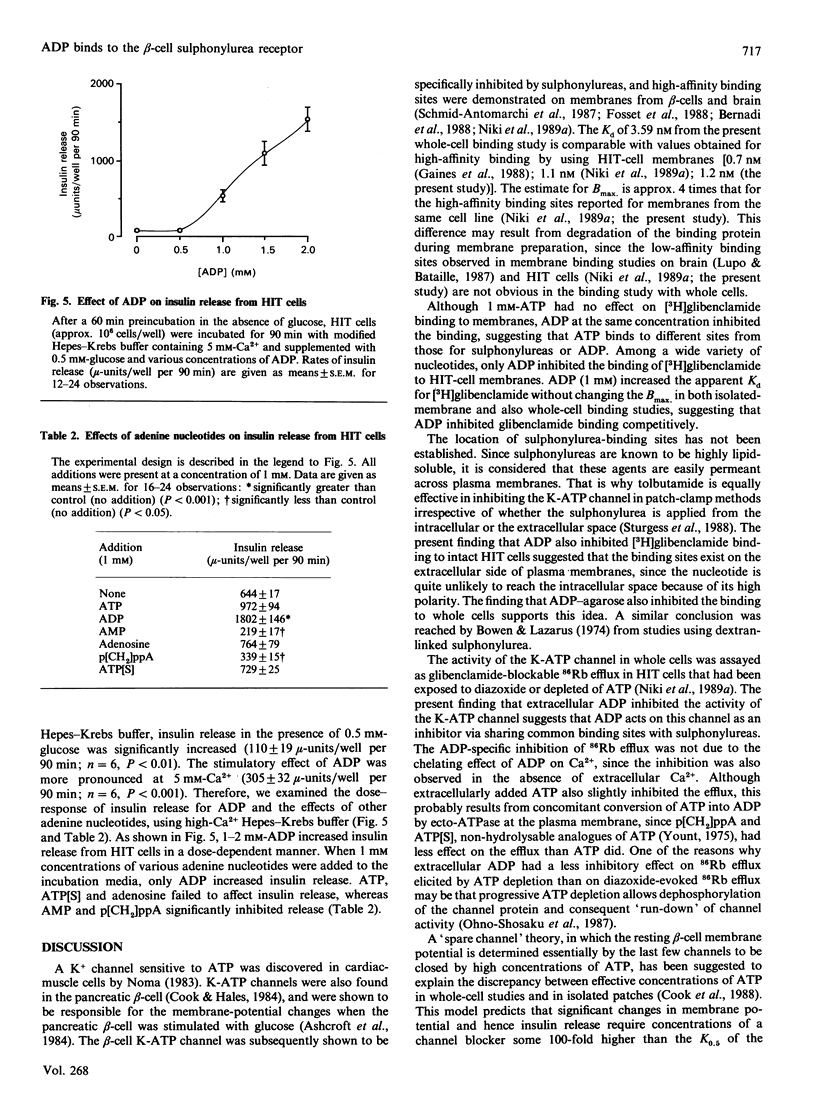
The effects of ADP on [3H]glibenclamide binding to membranes and whole cells, the activity of the ATP-sensitive K+ channel (K-ATP channel), intracellular Ca2+ concentration and insulin secretion were studied in a hamster pancreatic beta-cell line, HIT T15. ADP dose-dependently inhibited [3H]glibenclamide binding to membranes and to whole cells in a competitive manner. ADP-agarose also inhibited the binding to whole cells. The activity of the K-ATP channel was assayed by measuring 86Rb efflux from whole cells. ADP inhibited the 86Rb efflux elicited either by diazoxide or by ATP depletion. In the presence, but not in the absence, of extracellular Ca2+, ADP evoked a rapid and sustained increase in intracellular Ca2+ concentration as estimated with the fluorescent dye quin 2. Insulin release from HIT cells was also increased by 0.5-2 mM-ADP in the presence of 0.5 mM-glucose. These effects of ADP on glibenclamide binding, K-ATP channel activity and insulin release were specific for ADP, and were not reproduced by any other nucleotide so far tested. The present findings strongly suggest that ADP and sulphonylureas have common binding sites on the extracellular side of beta-cell plasma membranes, where they inhibit the activity of the K-ATP channel, resulting in an increase in intracellular Ca2+ concentration and insulin release.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft F. M. Adenosine 5'-triphosphate-sensitive potassium channels. Annu Rev Neurosci. 1988;11:97–118. doi: 10.1146/annurev.ne.11.030188.000525. [DOI] [PubMed] [Google Scholar]
- Ashcroft F. M., Harrison D. E., Ashcroft S. J. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. 1984 Nov 29-Dec 5Nature. 312(5993):446–448. doi: 10.1038/312446a0. [DOI] [PubMed] [Google Scholar]
- Ashcroft S. J., Crossley J. R. The effects of glucose, N-acetylglucosamine, glyceraldehyde and other sugars on insulin release in vivo. Diabetologia. 1975 Aug;11(4):279–284. doi: 10.1007/BF00422392. [DOI] [PubMed] [Google Scholar]
- Ashcroft S. J., Hammonds P., Harrison D. E. Insulin secretory responses of a clonal cell line of simian virus 40-transformed B cells. Diabetologia. 1986 Oct;29(10):727–733. doi: 10.1007/BF00870283. [DOI] [PubMed] [Google Scholar]
- Ashcroft S. J., Stubbs M. The glucose sensor in HIT cells is the glucose transporter. FEBS Lett. 1987 Jul 27;219(2):311–315. doi: 10.1016/0014-5793(87)80242-9. [DOI] [PubMed] [Google Scholar]
- Bernardi H., Fosset M., Lazdunski M. Characterization, purification, and affinity labeling of the brain [3H]glibenclamide-binding protein, a putative neuronal ATP-regulated K+ channel. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9816–9820. doi: 10.1073/pnas.85.24.9816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand G., Chapal J., Loubatieres-Mariani M. M. Potentiating synergism between adenosine diphosphate or triphosphate and acetylcholine on insulin secretion. Am J Physiol. 1986 Oct;251(4 Pt 1):E416–E421. doi: 10.1152/ajpendo.1986.251.4.E416. [DOI] [PubMed] [Google Scholar]
- Bowen V., Lazarus N. R. Insulin release from the perfused rat pancreas. Mode of action of tolbutamide. Biochem J. 1974 Aug;142(2):385–389. doi: 10.1042/bj1420385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cook D. L., Hales C. N. Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature. 1984 Sep 20;311(5983):271–273. doi: 10.1038/311271a0. [DOI] [PubMed] [Google Scholar]
- Cook D. L., Satin L. S., Ashford M. L., Hales C. N. ATP-sensitive K+ channels in pancreatic beta-cells. Spare-channel hypothesis. Diabetes. 1988 May;37(5):495–498. doi: 10.2337/diab.37.5.495. [DOI] [PubMed] [Google Scholar]
- Dunne M. J., Petersen O. H. Intracellular ADP activates K+ channels that are inhibited by ATP in an insulin-secreting cell line. FEBS Lett. 1986 Nov 10;208(1):59–62. doi: 10.1016/0014-5793(86)81532-0. [DOI] [PubMed] [Google Scholar]
- Fosset M., De Weille J. R., Green R. D., Schmid-Antomarchi H., Lazdunski M. Antidiabetic sulfonylureas control action potential properties in heart cells via high affinity receptors that are linked to ATP-dependent K+ channels. J Biol Chem. 1988 Jun 15;263(17):7933–7936. [PubMed] [Google Scholar]
- Gaines K. L., Hamilton S., Boyd A. E., 3rd Characterization of the sulfonylurea receptor on beta cell membranes. J Biol Chem. 1988 Feb 25;263(6):2589–2592. [PubMed] [Google Scholar]
- Hallam T. J., Rink T. J. Responses to adenosine diphosphate in human platelets loaded with the fluorescent calcium indicator quin2. J Physiol. 1985 Nov;368:131–146. doi: 10.1113/jphysiol.1985.sp015850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S. J., Ashcroft S. J. Effect of secretagogues on cytosolic free Ca2+ and insulin release in the hamster clonal beta-cell line HIT-T15. J Mol Endocrinol. 1988 Jul;1(1):13–17. doi: 10.1677/jme.0.0010013. [DOI] [PubMed] [Google Scholar]
- Kakei M., Kelly R. P., Ashcroft S. J., Ashcroft F. M. The ATP-sensitivity of K+ channels in rat pancreatic B-cells is modulated by ADP. FEBS Lett. 1986 Nov 10;208(1):63–66. doi: 10.1016/0014-5793(86)81533-2. [DOI] [PubMed] [Google Scholar]
- Lupo B., Bataille D. A binding site for [3H]glipizide in the rat cerebral cortex. Eur J Pharmacol. 1987 Aug 11;140(2):157–169. doi: 10.1016/0014-2999(87)90801-6. [DOI] [PubMed] [Google Scholar]
- Niki I., Ashcroft F. M., Ashcroft S. J. The dependence on intracellular ATP concentration of ATP-sensitive K-channels and of Na,K-ATPase in intact HIT-T15 beta-cells. FEBS Lett. 1989 Nov 6;257(2):361–364. doi: 10.1016/0014-5793(89)81572-8. [DOI] [PubMed] [Google Scholar]
- Niki I., Kelly R. P., Ashcroft S. J., Ashcroft F. M. ATP-sensitive K-channels in HIT T15 beta-cells studied by patch-clamp methods, 86Rb efflux and glibenclamide binding. Pflugers Arch. 1989 Oct;415(1):47–55. doi: 10.1007/BF00373140. [DOI] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983 Sep 8;305(5930):147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T., Zünkler B. J., Trube G. Dual effects of ATP on K+ currents of mouse pancreatic beta-cells. Pflugers Arch. 1987 Feb;408(2):133–138. doi: 10.1007/BF00581342. [DOI] [PubMed] [Google Scholar]
- Schmid-Antomarchi H., De Weille J., Fosset M., Lazdunski M. The receptor for antidiabetic sulfonylureas controls the activity of the ATP-modulated K+ channel in insulin-secreting cells. J Biol Chem. 1987 Nov 25;262(33):15840–15844. [PubMed] [Google Scholar]
- Sturgess N. C., Kozlowski R. Z., Carrington C. A., Hales C. N., Ashford M. L. Effects of sulphonylureas and diazoxide on insulin secretion and nucleotide-sensitive channels in an insulin-secreting cell line. Br J Pharmacol. 1988 Sep;95(1):83–94. doi: 10.1111/j.1476-5381.1988.tb16551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yount R. G. ATP analogs. Adv Enzymol Relat Areas Mol Biol. 1975;43:1–56. doi: 10.1002/9780470122884.ch1. [DOI] [PubMed] [Google Scholar]
- Zünkler B. J., Lins S., Ohno-Shosaku T., Trube G., Panten U. Cytosolic ADP enhances the sensitivity to tolbutamide of ATP-dependent K+ channels from pancreatic B-cells. FEBS Lett. 1988 Nov 7;239(2):241–244. doi: 10.1016/0014-5793(88)80925-6. [DOI] [PubMed] [Google Scholar]


