Abstract
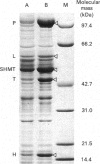
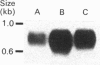
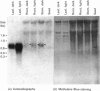
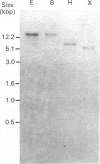
We have isolated and characterized cDNA clones encoding the H-protein of the glycine-cleavage system of pea (Pisum sativum) leaf mitochondria. The deduced primary structure revealed that the 131-amino-acid polypeptide is cytoplasmically synthesized with a 34-amino-acid mitochondrial targeting peptide. The lipoate-binding site was assigned to be lysine-63, as deduced from a sequence comparison with several lipoate-bearing proteins. The expression of the gene encoding H-protein was shown to occur specifically in the leaf tissue, with light exerting an additional effect by increasing the mRNA levels severalfold. Two polyadenylation sites were found in the mRNA, and a single-copy gene encoding the H-protein was detected in pea genome.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourguignon J., Neuburger M., Douce R. Resolution and characterization of the glycine-cleavage reaction in pea leaf mitochondria. Properties of the forward reaction catalysed by glycine decarboxylase and serine hydroxymethyltransferase. Biochem J. 1988 Oct 1;255(1):169–178. doi: 10.1042/bj2550169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford A. P., Aitken A., Beg F., Cook K. G., Yeaman S. J. Amino acid sequence surrounding the lipoic acid cofactor of bovine kidney 2-oxoglutarate dehydrogenase complex. FEBS Lett. 1987 Sep 28;222(1):211–214. doi: 10.1016/0014-5793(87)80221-1. [DOI] [PubMed] [Google Scholar]
- Dean C., Tamaki S., Dunsmuir P., Favreau M., Katayama C., Dooner H., Bedbrook J. mRNA transcripts of several plant genes are polyadenylated at multiple sites in vivo. Nucleic Acids Res. 1986 Mar 11;14(5):2229–2240. doi: 10.1093/nar/14.5.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douce R., Moore A. L., Neuburger M. Isolation and oxidative properties of intact mitochondria isolated from spinach leaves. Plant Physiol. 1977 Oct;60(4):625–628. doi: 10.1104/pp.60.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara K., Okamura-Ikeda K., Motokawa Y. Chicken liver H-protein, a component of the glycine cleavage system. Amino acid sequence and identification of the N epsilon-lipoyllysine residue. J Biol Chem. 1986 Jul 5;261(19):8836–8841. [PubMed] [Google Scholar]
- Fujiwara K., Okamura K., Motokawa Y. Hydrogen carrier protein from chicken liver: purification, characterization, and role of its prosthetic group, lipolic acid, in the glycine cleavage reaction. Arch Biochem Biophys. 1979 Oct 15;197(2):454–462. doi: 10.1016/0003-9861(79)90267-4. [DOI] [PubMed] [Google Scholar]
- Gardeström P., Bergman A., Ericson I. Oxidation of Glycine via the Respiratory Chain in Mitochondria Prepared from Different Parts of Spinach. Plant Physiol. 1980 Feb;65(2):389–391. doi: 10.1104/pp.65.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershwin M. E., Mackay I. R., Sturgess A., Coppel R. L. Identification and specificity of a cDNA encoding the 70 kd mitochondrial antigen recognized in primary biliary cirrhosis. J Immunol. 1987 May 15;138(10):3525–3531. [PubMed] [Google Scholar]
- Hartl F. U., Pfanner N., Nicholson D. W., Neupert W. Mitochondrial protein import. Biochim Biophys Acta. 1989 Jan 18;988(1):1–45. doi: 10.1016/0304-4157(89)90002-6. [DOI] [PubMed] [Google Scholar]
- Hummel K. B., Litwer S., Bradford A. P., Aitken A., Danner D. J., Yeaman S. J. Nucleotide sequence of a cDNA for branched chain acyltransferase with analysis of the deduced protein structure. J Biol Chem. 1988 May 5;263(13):6165–6168. [PubMed] [Google Scholar]
- Hurkman W. J., Tanaka C. K. Solubilization of plant membrane proteins for analysis by two-dimensional gel electrophoresis. Plant Physiol. 1986 Jul;81(3):802–806. doi: 10.1104/pp.81.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi C. P. Putative polyadenylation signals in nuclear genes of higher plants: a compilation and analysis. Nucleic Acids Res. 1987 Dec 10;15(23):9627–9640. doi: 10.1093/nar/15.23.9627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi G., Hiraga K. The mitochondrial glycine cleavage system. Unique features of the glycine decarboxylation. Mol Cell Biochem. 1982 Jun 25;45(3):137–149. doi: 10.1007/BF00230082. [DOI] [PubMed] [Google Scholar]
- Kochi H., Kikuchi G. Mechanism of the reversible glycine cleavage reaction in Arthrobacter globiformis. I. Purification and function of protein components required for the reaction. J Biochem. 1974 May;75(5):1113–1127. doi: 10.1093/oxfordjournals.jbchem.a130483. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lau K. S., Griffin T. A., Hu C. W., Chuang D. T. Conservation of primary structure in the lipoyl-bearing and dihydrolipoyl dehydrogenase binding domains of mammalian branched-chain alpha-keto acid dehydrogenase complex: molecular cloning of human and bovine transacylase (E2) cDNAs. Biochemistry. 1988 Mar 22;27(6):1972–1981. doi: 10.1021/bi00406a025. [DOI] [PubMed] [Google Scholar]
- Lütcke H. A., Chow K. C., Mickel F. S., Moss K. A., Kern H. F., Scheele G. A. Selection of AUG initiation codons differs in plants and animals. EMBO J. 1987 Jan;6(1):43–48. doi: 10.1002/j.1460-2075.1987.tb04716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Robinson J. R., Klein S. M., Sagers R. D. Glycine metabolism. Lipoic acid as the prosthetic group in the electron transfer protein P2 from Peptococcus glycinophilus. J Biol Chem. 1973 Aug 10;248(15):5319–5323. [PubMed] [Google Scholar]
- Saint-Blancard J., Fourcart J., Limonne F., Girot P., Boschetti E. Nouveaux échangeurs d'ions Trisacryl: intérêt et application au fractionnement des protéines du plasma humain. Ann Pharm Fr. 1981;39(5):403–409. [PubMed] [Google Scholar]
- Sarojini G., Oliver D. J. Extraction and partial characterization of the glycine decarboxylase multienzyme complex from pea leaf mitochondria. Plant Physiol. 1983 May;72(1):194–199. doi: 10.1104/pp.72.1.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer M. E., Darlison M. G., Stephens P. E., Duckenfield I. K., Guest J. R. Nucleotide sequence of the sucB gene encoding the dihydrolipoamide succinyltransferase of Escherichia coli K12 and homology with the corresponding acetyltransferase. Eur J Biochem. 1984 Jun 1;141(2):361–374. doi: 10.1111/j.1432-1033.1984.tb08200.x. [DOI] [PubMed] [Google Scholar]
- Stephens P. E., Darlison M. G., Lewis H. M., Guest J. R. The pyruvate dehydrogenase complex of Escherichia coli K12. Nucleotide sequence encoding the dihydrolipoamide acetyltransferase component. Eur J Biochem. 1983 Jul 1;133(3):481–489. doi: 10.1111/j.1432-1033.1983.tb07490.x. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. L., Oliver D. J. Light-induced increases in the glycine decarboxylase multienzyme complex from pea leaf mitochondria. Arch Biochem Biophys. 1986 Aug 1;248(2):626–638. doi: 10.1016/0003-9861(86)90517-5. [DOI] [PubMed] [Google Scholar]
- Wilkins T. A., Raikhel N. V. Expression of rice lectin is governed by two temporally and spatially regulated mRNAs in developing embryos. Plant Cell. 1989 May;1(5):541–549. doi: 10.1105/tpc.1.5.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman S. J., Fussey S. P., Danner D. J., James O. F., Mutimer D. J., Bassendine M. F. Primary biliary cirrhosis: identification of two major M2 mitochondrial autoantigens. Lancet. 1988 May 14;1(8594):1067–1070. doi: 10.1016/s0140-6736(88)91894-6. [DOI] [PubMed] [Google Scholar]
- Yeaman S. J. The 2-oxo acid dehydrogenase complexes: recent advances. Biochem J. 1989 Feb 1;257(3):625–632. doi: 10.1042/bj2570625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Scholl R., Browse J., Somerville C. Double stranded DNA sequencing as a choice for DNA sequencing. Nucleic Acids Res. 1988 Feb 11;16(3):1220–1220. doi: 10.1093/nar/16.3.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G., Steppuhn J., Herrmann R. G. Domain structure of mitochondrial and chloroplast targeting peptides. Eur J Biochem. 1989 Apr 1;180(3):535–545. doi: 10.1111/j.1432-1033.1989.tb14679.x. [DOI] [PubMed] [Google Scholar]