FIG 1.
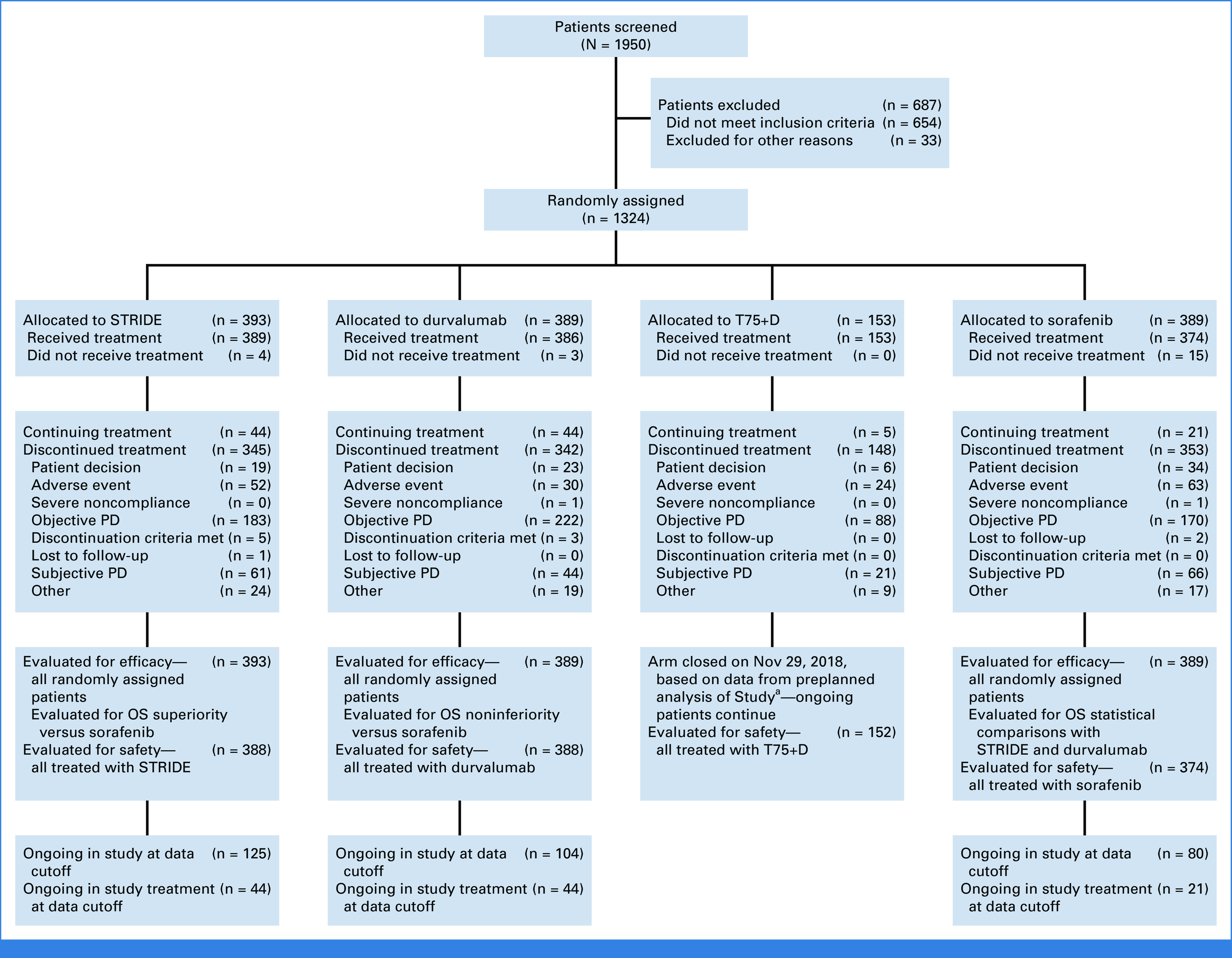
CONSORT diagram. aA preplanned analysis of Study 22 demonstrated that T75+D did not meaningfully differentiate from durvalumab monotherapy in terms of efficacy, and toxicity was slightly increased. Thus, enrollment to T75+D in HIMALAYA was closed on Nov 29, 2018. OS, overall survival; PD, progressive disease; STRIDE, Single Tremelimumab Regular Interval Durvalumab; T75+D, tremelimumab 75 mg once every 4 weeks for four doses plus durvalumab 1,500 mg once every 4 weeks.
