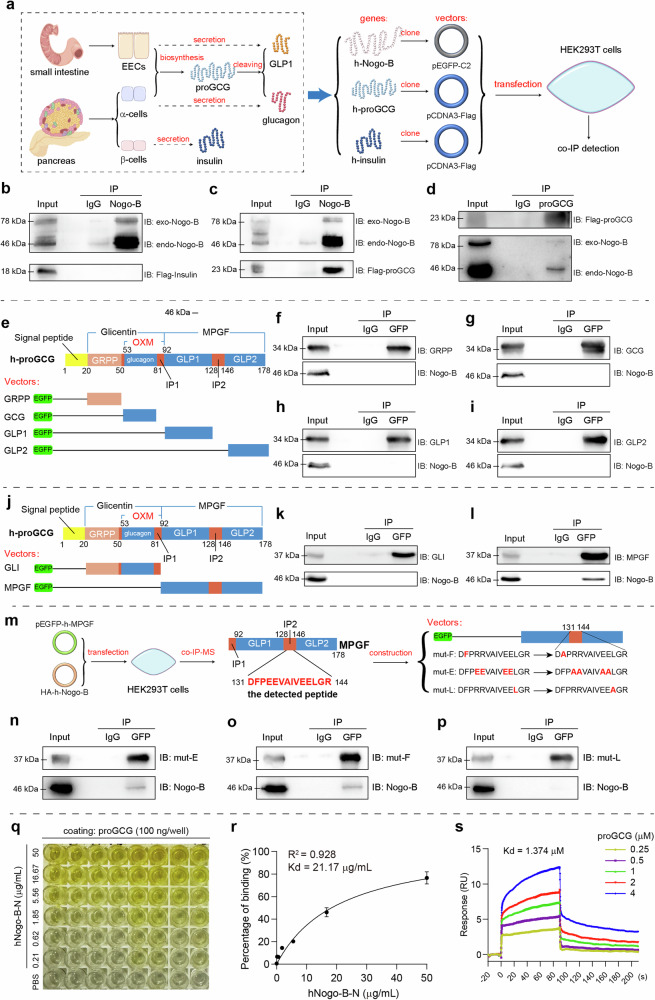
Fig. 3. Nogo-B interacts with proGCG.
a Tissue-specific post-translational modifications of glucagon and GLP1, and the strategy for the co-IP experiments. h in “h-Nogo-B, h-proGCG and h-insulin” means human-derived. b Immunoprecipitation assay with Nogo-B and exogenous insulin in HEK293T cells (n = 3 biological replicates). c, d Immunoprecipitation assay with Nogo-B and exogenous proGCG in HEK293T cells (n = 3 biological replicates). e Construction of EGFP-tagged expression vectors for proGCG-sheared basic short peptides (GRPP, glucagon, GLP1, and GLP2). f–i Immunoprecipitation assay with Nogo-B and proGCG-sheared basic short peptides (GRPP, glucagon, GLP1, and GLP2) (n = 3 biological replicates). j Construction of EGFP-tagged expression vectors for glicentin (GLI) and major proglucagon fragment (MPGF). k, l Immunoprecipitation assay with Nogo-B and GLI and MPGF (n = 3 biological replicates). m Mass spectrometric detection of the peptide bound to MPGF by Nogo-B, where the peptide with the highest secondary structure similarity to the profile was in the IP2 region. Construction of EGFP-tagged expression vectors for the MPFG with mutations in the hydrophobic amino acid site. n–p Immunoprecipitation assay with Nogo-B and MPFG with mutations in the hydrophobic amino acid site (n = 3 biological replicates). q ELISA for the binding of Nogo-B to proGCG. r Non-linear fit analysis of ELISA results (n = 6 biological replicates). s Affinity constants of Nogo-B and proGCG detected by SPR. All experiments of (b–d, f–I, k, l, n–p) were repeated at least three times Data are expressed as the mean ± SEM. Source data are provided as a Source Data file.