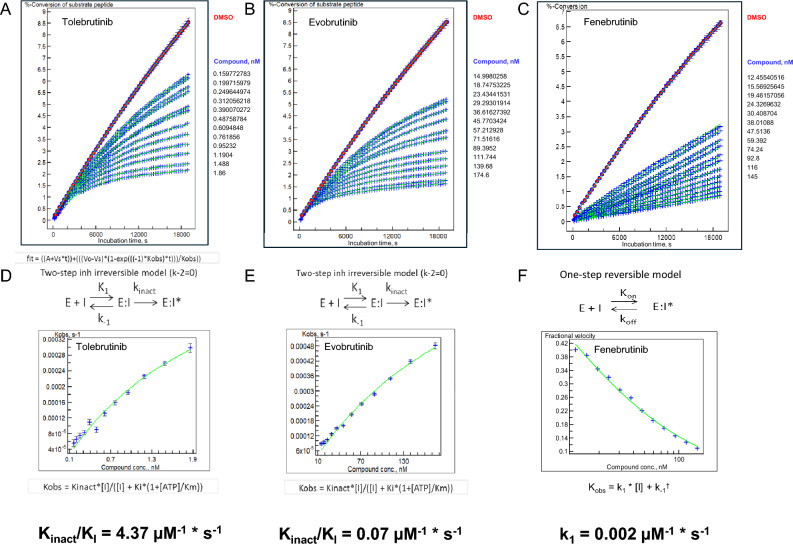
Fig. 1.
Microfluidic kinetic assay to measure BTK phosphotransferase activity as a function of inhibitor concentrations. a–c The observed rate of phosphokinase activity was estimated using a microfluidic assay coupled to electrophorectic separation of peptide substrate from phosphopeptide product. A total of 60 samples per well were collected at precisely determined times (~ 5-min intervals) over the course of ~ 315 min. Each data point represents the ratio of conversion of pseudosubstrate to product as a function of time from an individual well containing 1% DMSO (uninhibited enzyme reaction rate, n = 6), an enzyme-free reaction to establish 100% inhibition (n = 6), or the indicated concentration of inhibitor (n = 1 for each of 12 concentrations) displayed along the right margin for each panel. d–f The family of curves for each analyte were fitted using three models (one-step reversible, one-step irreversible, two-step irreversible), and the best-fit model was used to determine Kobs for each article. These data were then transformed using the best-fit model to determine Kinact/Ki (tolebrutinib and evobrutinib) or Ki (fenebrutinib) to estimate the relative reaction rates for each test article