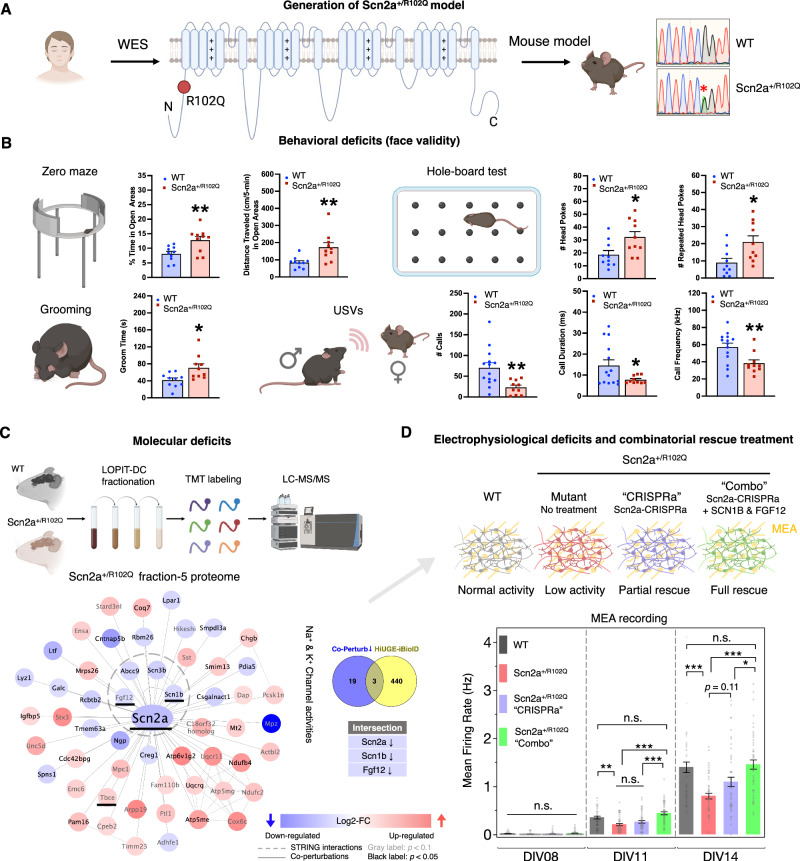
Fig. 3. Intersectional proteomics reveal hidden molecular mechanism of a patient-derived Scn2a mutation.
A Generation of a mouse model based on a clinically identified Scn2a missense mutation (R102Q) in autistic individuals. WES: whole exome sequencing. B Behavioral face-validity of Scn2a+/R102Q mutants was assessed by the zero maze as the percent time and distance traveled in the open areas; the hole-board test as numbers of head pokes and repeated head-pokes; the self-grooming test; and the ultrasonic vocalizations (USVs) as numbers of calls, call durations, and call frequencies during social interaction. No differences were detected in the metrics of pre-social (baseline) responses in the USV tests. *p < 0.05, **p < 0.01, WT vs. Scn2a+/R102Q mice; independent samples t-tests, two-tailed (n = 10 or 14 mice per group). Statistics are summarized in Supplementary Data S6. Plots are mean ± SEM. C Spatial proteomics reveals co-perturbations in Scn2a+/R102Q mutants, two-tailed heteroscedastic t-test on log2-transformed data. MCL analysis discovered a key cluster associated with voltage-gated channel activity that is downregulated, including three targets that intersect with the Scn2a HiUGE-iBioID proximity proteome (underlined). D Scn2a+/R102Q mutant neurons show attenuated activity with the MEA. Scn2a-CRISPRa treatment and a “Combo” treatment with additional expression of SCN1B and FGF12 show differential efficacy in restoring neural activity deficits. *p < 0.05; **p < 0.01; ***p < 0.001; n.s.: non-significant. One-way ANOVA followed by post-hoc Tukey HSD tests (n = 48 wells). Plots are mean ± SEM.