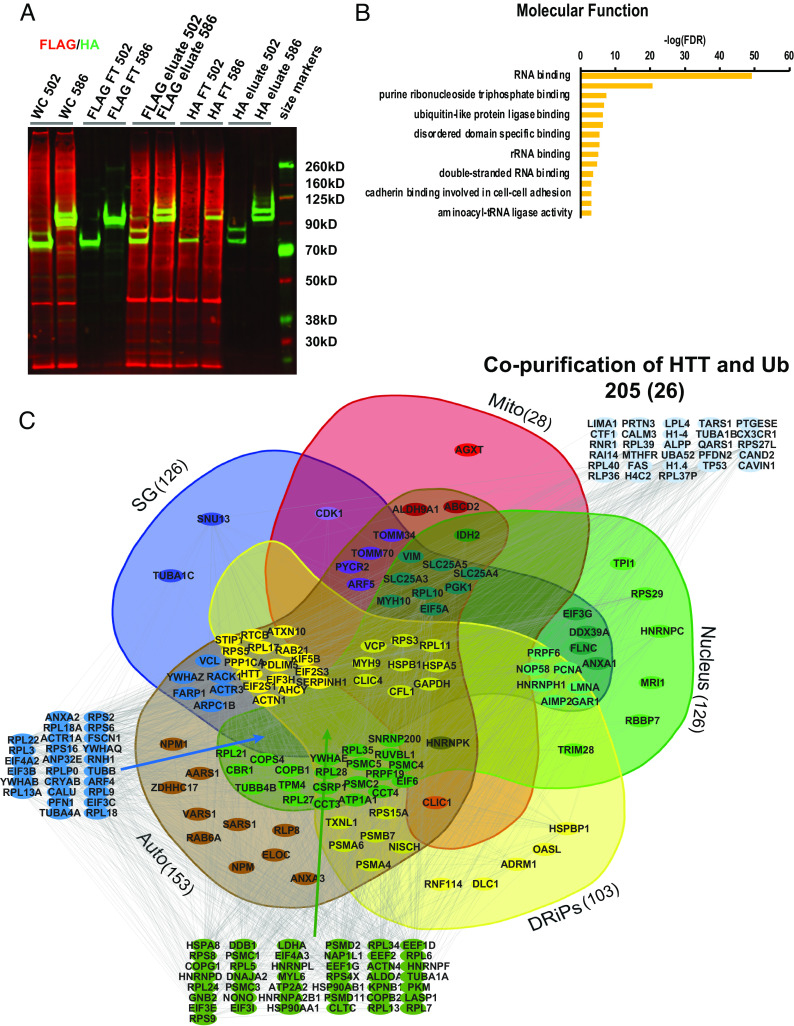
Fig. 3.
HTT copurifies with potentially ubiquitinated RNA-binding proteins. 17Q-502-HIS-HA-HA-HIS and FLAG-ubiquitin were coexpressed in St14A cells, crosslinked with formaldehyde, tandem copurified, and subjected to mass spectrometry analysis. (A) Plasmids encoding 17Q N-terminal 502 or 586 amino acid HTT fragments C-terminally tagged with HIS-HA-HA-HIS were transfected into St14A cells together with FLAG-ubiquitin. Cells were subjected to crosslinking with formaldehyde 2 d post-transfection, then lysed. Lysates were incubated overnight with an anti-FLAG matrix, washed then eluted with FLAG peptide, then incubated overnight with an anti-HA matrix, washed, and eluted with glycine pH 2. Samples from each copurification step were subjected to western analysis with anti-FLAG MAB (red) and polyclonal anti-HA antibody (green). FT denotes flow through of proteins that did not bind matrix. The final HA eluate shows bands the size of unmodified HTT fragment (green), and also a monoubiquitinated species (both red and green), together with copurifying ubiquitinated proteins (red). (B) Molecular function analysis of the ubiquitinated proteins associated noncovalently with HTT showed them to be most significantly RBPs. (C) 205 proteins copurified significantly above vector control in at least 2 of 3 tandem purifications. These proteins were found in protein databases for the nucleus (61%), stress granules (SG, 61%), defective ribosomal products (DRiPs, 50%), brain-derived autophagosomes (auto, 75%), and mitochondria (mito, 14%).