Fig. 4.
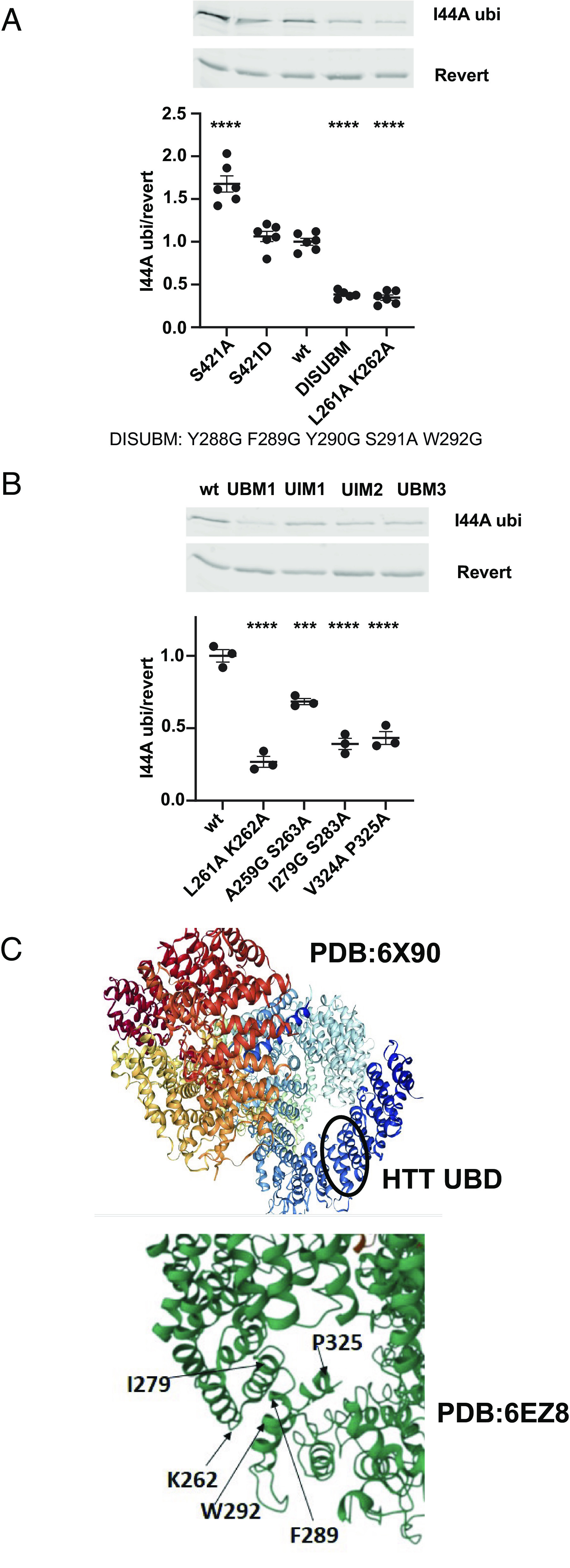
Mutation of amino acids within HTT’s 235-367 amino acid ubiquitin-binding domain destabilizes the in vitro interaction with I44A ubiquitin. (A, B) HTT GST-235-502 fragment was incubated in vitro with I44A ubiquitin. DISUBM (Y288G F289G Y290G S291A W292G) and UBM1 (L261A K262A), UIM1 (A259G S263A), UIM2 (I279G S283A), and UBM3 (V324A P325A) mutations reduced the in vitro interaction between I44A ubiquitin and HTT, while S421A enhanced the interaction as analyzed by One-Way ANOVA with Bonferroni’s multiple comparisons test and compared with wt control. (C) Cryo-EM structure from PDB:6X90 (46) or 6EZ8 (47) demonstrates the domain (residues 261 to 325, black circle) and amino acids involved in the in vitro HTT interaction with I44A ubiquitin.
