| Code/trivial name a | IUPAC name/SMILES notation/InChiKey b | Structural formula b |
|---|---|---|
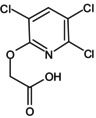
| Triclopyr |
[(3,5,6‐trichloro‐2‐pyridyl)oxy]acetic acid O=C(O)COC1 = NC(Cl) = C(Cl)C=C1Cl REEQLXCGVXDJSQ‐UHFFFAOYSA‐N |
|
|
Triclopyr‐butotyl (Triclopyr‐BEE) (Triclopyr butoxyethyl ester) (BEE) |
2‐butoxyethyl [(3,5,6‐trichloro‐2‐pyridyl)oxy]acetate O=C(OCCOCCCC)COC1 = NC(Cl) = C(Cl)C=C1Cl IVDRCZNHVGQBHZ‐UHFFFAOYSA‐N |

|
|
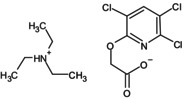
triclopyr triethylammonium (TEA) (Triclopyr‐TEA) |
triethylammonium [(3,5,6‐trichloro‐2‐pyridyl)oxy]acetate O=C([O‐])COC1 = NC(Cl) = C(Cl)C=C1Cl.CC[NH+](CC)CC ROKVVMOXSZIDEG‐UHFFFAOYSA‐N |
|
|
3,5,6‐TCP (3,5,6‐trichloro‐2‐pyridinol) (TCP) |
3,5,6‐trichloropyridin‐2‐ol OC1 = NC(Cl) = C(Cl)C=C1Cl WCYYAQFQZQEUEN‐UHFFFAOYSA‐N |

|
|
3,5,6‐TCP Glucuronide |
3,5,6‐trichloropyridin‐2‐yl D‐glucopyranosiduronic acid ClC1 = C(Cl)C=C(Cl)C(OC2[C@@H]([C@H]([C@@H]([C@@H](C(O) = O)O2)O)O)O) = N1 XIPKXSPRDJUGPJ‐OVSONBGKSA‐N |

|
|
3,5,6‐TCP Sulfate |
3,5,6‐trichloropyridin‐2‐yl hydrogen sulfate ClC1 = C(Cl)C=C(Cl)C(OS(=O)(O) = O) = N1 VTTUBUMMKMLULX‐UHFFFAOYSA‐N |

|
|
3,6‐DCP (3,6‐dichloro‐2‐pyridinol) |
3,6‐dichloropyridin‐2‐ol ClC1 = CC=C(N=C1O)Cl UGPDKBDRRLFGFD‐UHFFFAOYSA‐N |

|
|
6‐MCP (6‐chloro‐2‐pyridinol) (MCP) (Monochloro‐2‐pyridinol) |
6‐chloropyridin‐2‐ol OC1 = NC(Cl) = CC=C1 CLNNBQDAAGDAHI‐UHFFFAOYSA‐N |

|
| TMP (3,5,6‐trichloro‐2‐methoxypyridine) |
2,3,5‐trichloro‐6‐methoxypyridine ClC1 = CC(Cl) = C(N=C1OC)Cl RLIVUWLXZBDMBL‐UHFFFAOYSA‐N |

|
| DCA ([(3,6‐Dichloropyridin‐2‐yl)oxy] acetic acid) |
2‐((3,6‐dichloropyridin‐2‐yl)oxy)acetic acid O=C(O)COC1 = NC(Cl) = CC=C1Cl KOSARFJJBUXYNE‐UHFFFAOYSA‐N |

|
| maleamic acid |
(Z)‐4‐amino‐4‐oxobut‐2‐enoic acid O=C(O)/C=C\C(N) = O FSQQTNAZHBEJLS‐UPHRSURJSA‐N |

|
| fumaric amide |
(E)‐4‐amino‐4‐oxobut‐2‐enoic acid O=C(O)/C=C/C(N) = O FSQQTNAZHBEJLS‐OWOJBTEDSA‐N |

|
| succinamic acid |
4‐amino‐4‐oxobutanoic acid O=C(O)CCC(N) = O JDVPQXZIJDEHAN‐UHFFFAOYSA‐N |

|
| succinic acid |
butanedioic acid O=C(O)CCC(O) = O KDYFGRWQOYBRFD‐UHFFFAOYSA‐N |

|
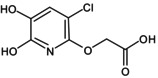
| ((3‐chloro,5,6 dihydroxy − 2‐pyridinyl)oxy)acetic acid |
2‐((3‐chloro‐5,6‐dihydroxypyridin‐2‐yl)oxy)acetic acid O=C(O)COC1 = NC(O) = C(O)C=C1Cl SALNNZHUWLJLBD‐UHFFFAOYSA‐N |
|
| chloromaleamic acid |
(E)‐4‐amino‐3‐chloro‐4‐oxobut‐2‐enoic acid O=C(O)/C=C(Cl)\C(N) = O ZJSYFBCDJYEWPY‐OWOJBTEDSA‐N |

|
| fumaric acid |
(2E)‐but‐2‐enedioic acid O=C(O)/C=C/C(O) = O VZCYOOQTPOCHFL‐OWOJBTEDSA‐N |

|
| chlorofumaric amide |
(Z)‐4‐amino‐2‐chloro‐4‐oxobut‐2‐enoic acid NC(/C=C(Cl)/C(O) = O) = O LAZUBSHZGQELHK‐UPHRSURJSA‐N |

|
| deschloro TCP sulfonic acid |
2,5‐dichloro‐6‐hydroxypyridine‐3‐sulfonic acid OS(=O)(=O)c1cc(Cl)c(O)nc1Cl UFXGVNDRVPSLTR‐UHFFFAOYSA‐N |
or isomer |
| deschloro TMP sulfonic acid |
2,5‐dichloro‐6‐methoxypyridine‐3‐sulfonic acid OS(=O)(=O)c1cc(Cl)c(OC)nc1Cl ITRDYWURDCYPBJ‐UHFFFAOYSA‐N |
or isomer |
The metabolite name in bold is the name used in the conclusion.
ACD/Name 2019.1.1 ACD/Labs 2019 Release (File version N05E41, Build 110555, 18 July 2019).
ACD/ChemSketch 2019.1.1 ACD/Labs 2019 Release (File version C05H41, Build 110712, 24 July 2019).


