Abstract
Aims
Atrial fibrillation (AF), the most prevalent clinical arrhythmia, is associated with atrial remodelling manifesting as acute and chronic alterations in expression, function, and regulation of atrial electrophysiological and Ca2+-handling processes. These AF-induced modifications crosstalk and propagate across spatial scales creating a complex pathophysiological network, which renders AF resistant to existing pharmacotherapies that predominantly target transmembrane ion channels. Developing innovative therapeutic strategies requires a systems approach to disentangle quantitatively the pro-arrhythmic contributions of individual AF-induced alterations.
Methods and results
Here, we built a novel computational framework for simulating electrophysiology and Ca2+-handling in human atrial cardiomyocytes and tissues, and their regulation by key upstream signalling pathways [i.e. protein kinase A (PKA), and Ca2+/calmodulin-dependent protein kinase II (CaMKII)] involved in AF-pathogenesis. Populations of atrial cardiomyocyte models were constructed to determine the influence of subcellular ionic processes, signalling components, and regulatory networks on atrial arrhythmogenesis. Our results reveal a novel synergistic crosstalk between PKA and CaMKII that promotes atrial cardiomyocyte electrical instability and arrhythmogenic triggered activity. Simulations of heterogeneous tissue demonstrate that this cellular triggered activity is further amplified by CaMKII- and PKA-dependent alterations of tissue properties, further exacerbating atrial arrhythmogenesis.
Conclusions
Our analysis reveals potential mechanisms by which the stress-associated adaptive changes turn into maladaptive pro-arrhythmic triggers at the cellular and tissue levels and identifies potential anti-AF targets. Collectively, our integrative approach is powerful and instrumental to assemble and reconcile existing knowledge into a systems network for identifying novel anti-AF targets and innovative approaches moving beyond the traditional ion channel-based strategy.
Keywords: Systems biology, Computational biology, Physiology, Population modelling, Electrophysiology, Upstream signalling, Arrhythmias, Atrial fibrillation
Graphical Abstract
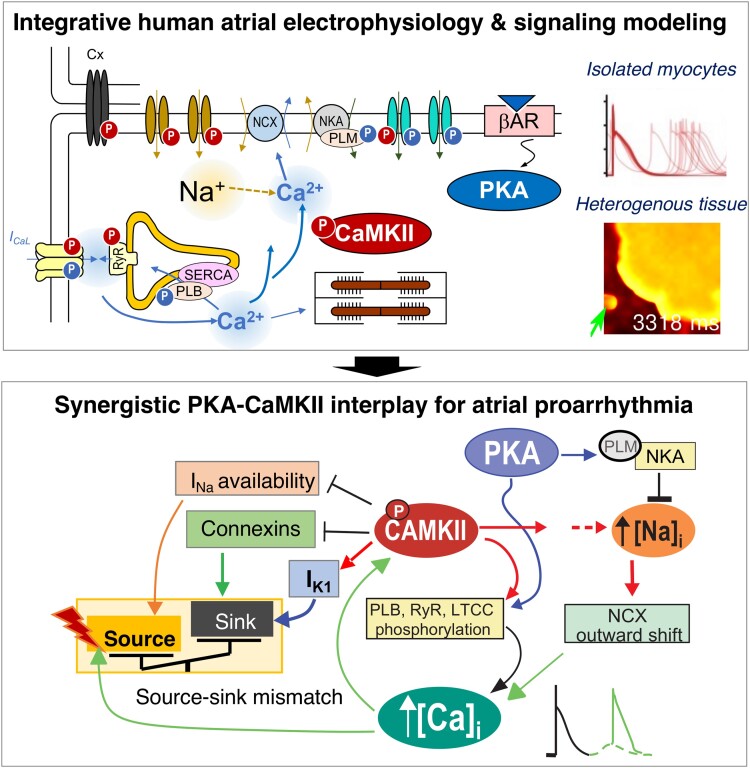
Graphical Abstract.
A novel integrative human atrial electrophysiology and signalling model reveals synergistic PKA and CaMKII interactions promoting delayed after depolarizations and triggered action potentials in both atrial myocytes and tissues. CaMKII, Ca2+/CaM-dependent protein kinase II; Cx, connexin; IK1, inward rectifier K+ current; INa, fast Na+ current; ICaL, L-type Ca2+ current; NCX, Na+/Ca2+ exchanger; NKA, Na+/K+ ATPase; PLB, phospholamban; PLM, phospholemman; RyR, ryanodine receptor 2; SERCA, sarco/endoplasmic reticulum Ca2+ ATPase; βAR, β adrenergic receptor; PKA, protein kinase A.
Time of primary review: 22 days
1. Introduction
Atrial fibrillation (AF), characterized by irregular and rapid activation of the upper chambers of the heart, is the most frequently encountered arrhythmia, and its prevalence is increasing worldwide.1–3 AF is associated with underlying cardiac comorbidities and with increased risks of stroke, heart failure, and mortality, thus posing significant health and socio-economic burden.1–6 Existing strategies for treating AF, such as rate control and rhythm control through anti-arrhythmic drugs (typically, ion channel blockers) or catheter-based ablation,2 suffer from unsatisfactory efficacy and adverse effects.4 The challenges and obstacles hampering the development of novel therapeutic approaches are underscored by the complex pathological mechanisms underlying AF, which are multi-factorial and involve electrical remodelling, Ca2+-handling abnormalities, structural, and neurohormonal changes.3,7–9 These complex multi-level alterations have been mechanistically linked to ectopic (triggered) activity and impulse re-entry in cardiac tissue, which can initiate and sustain arrhythmia in the atria, thus leading to AF.3,7,9,10
Cardiomyocyte and tissue responses to stressors are mediated by an intricate signalling network that allows the heart to adapt and meet physiological needs. Among these signals, protein kinase A (PKA) and Ca2+/calmodulin-dependent protein kinase II (CaMKII) are two protein kinases that phosphorylate a vast array of ion channels and Ca2+-handling and regulatory proteins and play critical roles in fine-tuning atrial cardiomyocyte stress responses.11–27 These involve changes in transmembrane potential homeostasis via both direct influences on sarcolemmal ion channels and transporters, as well as indirect changes in Ca2+ signalling that acutely regulate transmembrane fluxes and can lead to remodelling in the chronic (pathologic) setting.13,18,20–23,25,28 Indeed, dysregulated PKA and CaMKII signalling have emerged as key transducers of myocardial stress responses to increased arrhythmia propensity via both acute and chronic regulation of cardiac structure and function and as potential novel targets for ‘upstream’ anti-arrhythmic therapy.29,30 Since PKA and CaMKII signalling share several downstream targets in the heart, and PKA-mediated Ca2+ elevation is a well-established mode of CaMKII activation, these two signalling pathways are strongly interrelated and may crosstalk to collectively promote arrhythmogenesis.31 However, most previous experimental work investigated these signals in isolation, studied their separate contribution to promote arrhythmia triggers, and led to controversies on the relative roles of PKA- and CaMKII-dependent processes in cardiac dysfunction and arrhythmogenesis.32 In addition, most of these studies are performed in isolated cardiomyocytes, and whether and how PKA and CaMKII signalling affect tissue properties to cause arrhythmia remain poorly understood. Therefore, we contend that developing ‘upstream’ therapeutic strategies focusing on these two signalling pathways requires (i) untangling the complex temporal regulations of PKA and CaMKII signalling cascades and dissecting contributions from modifications of each signalling target and (ii) integrating the observations across spatial scales to reveal how subcellular- and cellular-level alterations interact with complex cardiac tissue dynamics.
While these are challenging experimental goals, mechanistic computational modelling of cardiomyocytes has proven instrumental to understand the complex interplay of membrane potential and Ca2+-dependent signalling, by not only integrating functional and structural experimental (and clinical) data but also revealing experimentally unrecognized mechanistic underpinnings of physiological and pathophysiological processes.31,33–36 Indeed, extensive previous studies have constructed biochemically detailed models of PKA or CaMKII signalling and integrated these formulations with ventricular electrophysiology and Ca2+-signalling models to provide insights into their individual roles in regulating ventricular excitation-contraction (EC) coupling and arrhythmia in health and disease, namely heart failure.31,37–40 However, cell ultrastructure, subcellular Ca2+-signalling, and EC coupling differ between atrial and ventricular cardiomyocytes,36,41–43 and appropriate computational models and studies are conspicuously absent in atrial physiology and pathophysiology. Integrating detailed descriptions of PKA and CaMKII signalling with biophysical models of electrophysiology and Ca2+ handling allows to study the independent and combined effects of these two important signals. Furthermore, the existing models describe average behaviours and do not typically account for parameter variabilities that may otherwise reflect key intercellular and inter-subject heterogeneities and do not include tissue level simulations, which may lend important insight into PKA and CaMKII interactions in affecting cardiac tissue parameters, ultimately causing arrhythmia.
In this study, we integrated contemporary knowledge of atrial electrophysiology, Ca2+ handling, and PKA and CaMKII signalling into a comprehensive multi-scale model framework to uncover novel mechanistic insights into atrial arrhythmogenesis by addressing the following questions:
Do PKA and CaMKII signalling act synergistically to create arrhythmogenic triggered activity in human atrial cardiomyocytes and tissue?
What are the mechanistic determinants of increased triggered activity at the subcellular, cellular, and tissue level?
What is the contribution of subcellular and cellular variability to triggered activity?
Early and delayed afterdepolarizations (EADs and DADs, respectively) underlie ectopic (triggered) activity, which is a major arrhythmogenic mechanism of AF.3,10,44 Here, we specifically focused on DADs, since DAD-induced triggered activity appears key to the spontaneous initiation of AF, whereas the contribution of EADs to atrial arrhythmogenesis is less clear,3 except of mutations hampering repolarization reserve as with long-QT syndrome.45
2. Methods
We coupled our well-established model of human atrial cardiomyocyte electrophysiology and Ca2+ handling46,47 with biochemically detailed systems models of CaMKII and adrenergic receptor (βAR)/cyclic adenosine monophosphate (cAMP)/PKA signalling pathways31,39,40,48–50 to build a novel integrative model of human atrial cardiomyocytes (see Supplementary material online, Figure S1). Each of the modules was updated to recapitulate new and human atrial-specific features. Namely, the human atrial electrophysiology and Ca2+-handling model was modified to incorporate a new Markovian formulation of L-type Ca2+ current (ICaL, Supplementary material online, Figure S22) based on previous work,51 add descriptions of atrial-predominant 2-pore and small-conductance Ca2+ dependent K+ currents (IK2P and IKCa), and update model formulations of rapidly and slowly activating delayed rectifier K+ currents (IKr and IKs), inward rectifier K+ current (IK1), background Cl− current (IClB), fast and late Na+ current (INa and INaL). CaMKII and PKA signalling models were also extended to incorporate dynamic functional effects on additional downstream targets/substrates, including dynamic descriptions of CaMKII-dependent regulations of gap junction conductance52,53 and atrial-predominant IKur20 (see Supplementary material online, Table S4). The resulting human atrial cardiomyocyte integrative model was parameterized (model maximum ion channel conductances and transport rates were adjusted, see Supplementary material online, Table S5) to recapitulate key dynamic behaviours of human atrial cardiomyocyte AP and Ca2+ at various pacing rates (see Supplementary material online, Figure S2A–C) and rigorously validated by demonstrating its capability to reproduce characteristic responses of human atrial cardiomyocytes to a wide range of stressors and physiological challenges (see Supplementary material online, Figures S3–S8). We constructed populations of models39,54–58 of atrial myocytes and heterogeneous atrial tissue (see Supplementary material online, Supplementary Methods) to assess the precise contribution of PKA and CaMKII signalling to triggered activity in both atrial and pulmonary vein (PV)-like myocytes. We applied multivariate linear regression-based sensitivity analysis39,56–59 to gain quantitative understanding of influences of model parameters on key dynamic properties of atrial cardiomyocytes and performed logistic regression analysis55 to link model parameters to the occurrence of triggered activity. Detailed descriptions of model extensions, sensitivity analysis, tissue modelling, simulation protocols, and model implementation are provided in Supplementary material online, SupplementaryMethods.
2.1. Code availability
All our source codes and related parameter perturbations for building populations as well as sensitivity analyses used in this study are available for download at elegrandi.wixsite.com/grandilab/downloads and github.com/drgrandilab.
3. Results
3.1. Integrative systems models of electrophysiology, Ca2+ handling, and CaMKII and PKA signalling recapitulate key dynamics of human atrial electrophysiology and Ca2+ handling
To facilitate the quantitative assessment of the interplay between PKA and CaMKII in human atria, we constructed a novel integrative model that couples our well-established systems model of electrophysiology and Ca2+ signalling46 with biochemically detailed systems models of CaMKII and PKA signalling cascades31,40,49,50 (see Supplementary material online, Figure S1). This integrative framework has been previously established for rabbit,40 mouse,31 and human39, 60 ventricular cardiomyocytes but is currently lacking in contemporary models of human atrial cardiomyocytes.46,61–63
Our newly integrated model recapitulates the morphological characteristics and a wide range of physiological behaviours of action potential (AP) and Ca2+ transient (CaT) of human atrial cells documented in previous experiments (see Supplementary material online, Figure S2). Typical APs, CaT, and L-type Ca2+-current (ICaL) during the AP stimulated at various frequencies are shown in Supplementary material online, Figure S2Ai–iii. Our integrative cell model displays a typical Type-1 human atrial AP morphology that was most frequently encountered in experiments in human atria64 (see Supplementary material online, Figure S2Ai). Notably, simulated AP morphology and duration (APD) and systolic and diastolic Ca2+ levels, associated with cardiomyocyte contractile function, are markedly rate-dependent over a wide range of physiological pacing frequencies and well match with experimental recordings65–72 (see Supplementary material online, Figure S2Ai–ii and Figure S3A top row). Specifically, our model displays a hallmark positive APD-pacing cycle length (PCL) relationship (see Supplementary material online, Figure S2B), which is well established despite large variabilities among experimental findings, and the computed CaT amplitude vs. PCLs displays a biphasic relationship that closely resembles the documented intra-cellular CaT measurements by aequorin light signals71 and twitch force measurements72 at various pacing rates (see Supplementary material online, Figure S2C). Our model shows that activating PKA signalling by simulated application of 100 nM isoprenaline (ISO) slightly prolonged the AP, in agreement with experimental reports from human atrial myocytes.73,74 Our simulations also recapitulate the well-known effects of PKA signalling on cardiomyocyte Ca2+ handling, in that application of ISO increased ICaL and CaT amplitude, while accelerating CaT decay in both current (see Supplementary material online, Figure S2D) and voltage (see Supplementary material online, Figure S7) clamp settings,22 and the measured effects of CaMKII inhibition on the AP upstroke velocity and CaT characteristics (see Supplementary material online, Figure S8Ai and Bi). We validated our model by verifying the ability of our model to recapitulate characteristic dynamic responses of human atrial cardiomyocytes to various physiological stressors, pharmacological perturbation, and stimulation protocols (see Supplementary material online, Figures S3–S8). Detailed descriptions of model validation are provided in Supplementary material online, Supplementary Text.
3.2. Populations of human atrial cardiomyocyte models reveal a synergistic interplay between PKA and CaMKII in promoting cellular triggered activity
Originally proposed for neuroscience research,75,76 the populations-of-models approach has been widely applied to understand the uncertainty of modelling outcomes, calibrate the populations to physiologically relevant variabilities, and gain mechanistic understanding through sensitivity analyses.39,54–58,77 Using our validated integrative model, we generated populations of models to assess the roles of PKA and CaMKII signalling in promoting the propensity of atrial cells to develop DADs and triggered APs (tAPs). We created three different populations (size of 600) by randomly perturbing the model parameters (log-normal distribution of σ = 0.1) describing: (i) maximum ion channel conductances or transporter rates (Population-1, see Supplementary material online, Table S1), (ii) steady-state phosphorylation levels of PKA or CaMKII downstream targets (Population-2, see Supplementary material online, Table S2), and (iii) concentrations of proteins (e.g. protein phosphatases, phosphodiesterases, etc.) that are intermediates within the two signalling cascades and fine-tune the target phosphorylation (Population-3, see Supplementary material online, Table S3, similar to54). Building these populations allows for regression-based sensitivity analyses to understand how AP and CaT characteristics are affected by the model parameters (see Supplementary material online, Supplementary Text and Figure S9), providing solid foundations for applying these populations of models to investigate the precise dependence of AP- and CaT-related abnormalities on each model parameter.
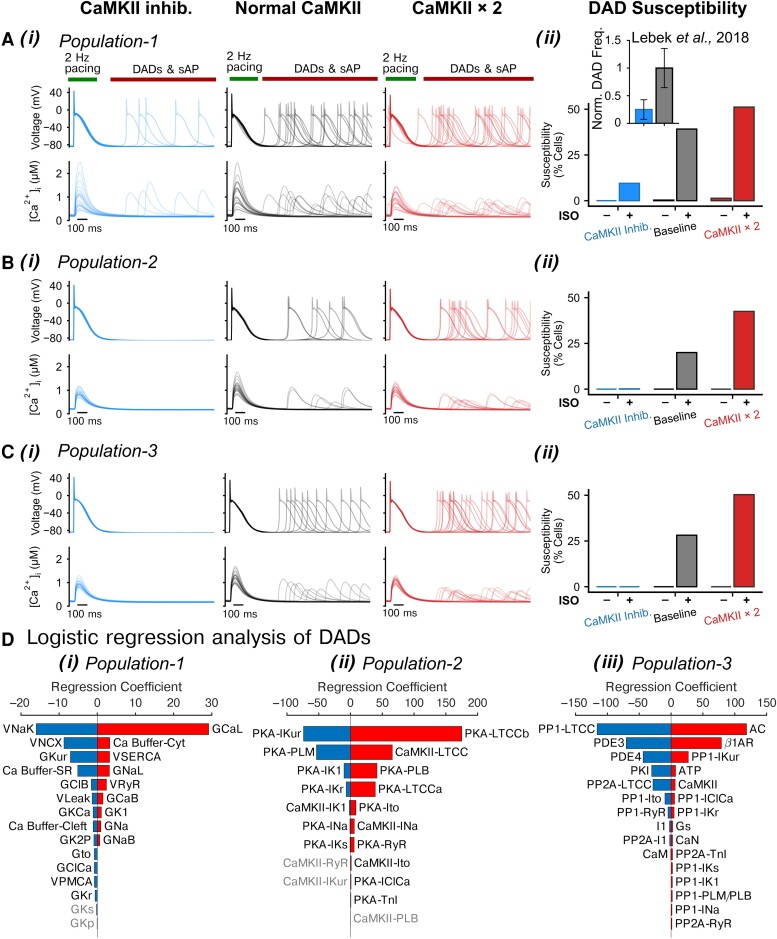
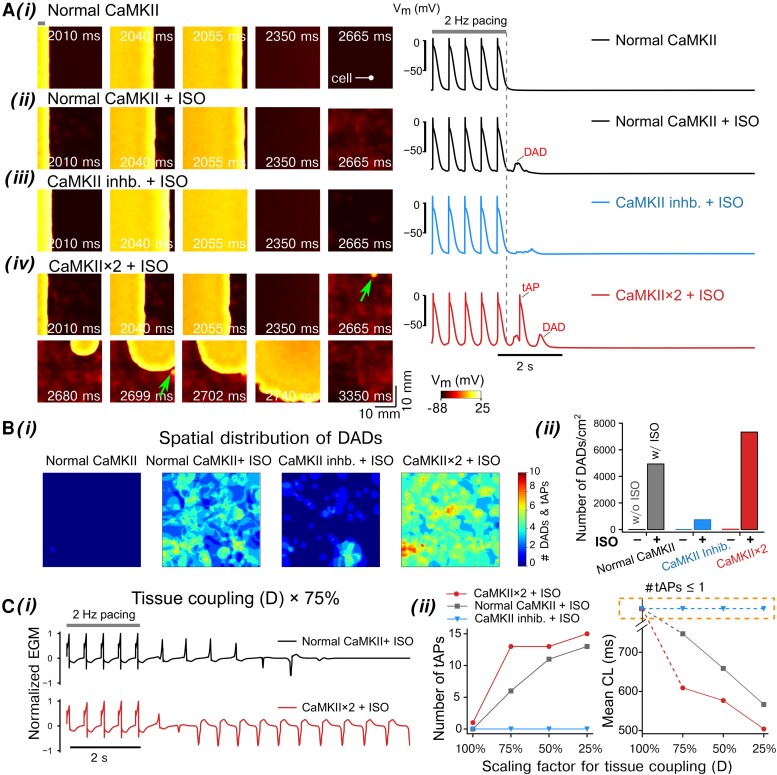
To quantify the contributions of PKA and CaMKII to atrial cardiomyocyte propensity to triggered activity, we subjected the three populations to various CaMKII settings (normal CaMKII, or increased CaMKII expression by two-fold, i.e. analogous to AF settings,12,18,20,22 and CaMKII inhibition) with (ISO, 100 nM) or without PKA activation. We investigated the propensity for DADs following a 2 Hz pacing-pause protocol (Figure 1A–C11; Supplementary material online, Figure S10). Our simulations in the control conditions rarely displayed DADs or abnormal [Ca2+]i (see Supplementary material online, Figure S10), whereas DAD incidence became substantial following simulated ISO application and further increased with CaMKII over-expression (by two-fold, i.e. CaMKII × 2, Figure 1A–C). Importantly, CaMKII inhibition dramatically suppressed the cellular arrhythmic events, uncovering a critical role of CaMKII in determining the propensity to cellular triggered activity. Importantly, our simulated effects of CaMKII inhibition on DAD incidence match well with experimental observations (Figure 1Aii inset), further validating our integrative model as a reliable tool for CaMKII studies.
Figure 1.
Populations of models uncover synergistic interplay between PKA and CaMKII signalling in promoting DADs. (A–C) (i) APs and CaTs simulated using (A) Population-1, (B) Population-2, and (C) Population-3 under CaMKII inhibition, normal CaMKII, or with two-fold CaMKII expression conditions after application of ISO following a 2 Hz pacing and pause protocol; (ii) fraction of cells developed DADs. Inset in (A) (ii): normalized frequency of DADs for human atrial cardiomyocytes under control vs. CaMKII inhibition conditions reported in experiments (Lebek et al.78). (D) Logistic regression analysis of DAD incidence under two-fold CaMKII expression conditions and after ISO application for (i) Population-1, (ii) Population-2, and (iii) Population-3, respectively. Please refer to Supplementary material online, Tables S1–S3 for abbreviation descriptions for panel (D).
Quantification of DAD events in the various populations and groups reveals a synergistic interplay between the PKA and CaMKII signalling in promoting cellular triggered activity (Figure 1Aii–Cii). Indeed, PKA signalling potentiated the pro-arrhythmic effects of CaMKII in atrial cardiomyocytes. The synergistic crosstalk between PKA and CaMKII resulted in the occurrence of DADs in 43–51% of cells in simulations with ISO and CaMKII × 2. Furthermore, CaMKII inhibition generally abolished DADs even in the presence of ISO, suggesting that CaMKII activation is required for the induction of cellular triggered activity, consistent with previous studies in engineered ventricular tissue.79
3.3. Logistic regression uncovers the key subcellular determinants of cellular triggered activity
Experimental investigations of triggered activity in human80 and rabbit81 atrial myocytes show a large degree of cell-to-cell variability. Likewise, our simulations uncovered intrinsic variabilities in the cellular susceptibility to develop triggered activity within each model population, whereby a fraction of cells displayed arrhythmic events, whereas many others did not. To quantitatively dissect the mechanisms underlying the variable susceptibility to triggered activity and arrhythmia, we performed logistic regression analyses55 to link the arrhythmic events to the parameters describing subcellular processes and signalling. Specifically, we focused on the simulations with both ISO application and two-fold CaMKII expression, where the size of cell subpopulation displaying DADs was comparable to that of the stable cell subpopulation, and applied binary coding to represent the presence/absence of cellular triggered activity. The sensitivity coefficients from the logistic regression analyses shed light on the influences of model parameters on arrhythmia propensity, with pro-arrhythmic processes being associated with positive coefficients and anti-arrhythmic processes with negative coefficients, and the coefficient magnitude informing the degree of parameter influence on the arrhythmic outcome.55 The values of the sensitivity coefficients provide quantitative understanding of how changing each individual process to the same extent affects the probability of the event of interest without assuming specific disease states. While the coefficients should not be interpreted as contributions in AF, the actual pro-arrhythmic impact in disease can be approximated by the product combining the coefficient and the disease-associated change of any given process, as further explained in Supplementary material online, Supplementary Text.
Logistic regression analysis uncovers the effects of altering each underlying ionic and signalling process on the cellular propensity to DADs and tAPs (Figure 1D). Comparing the outcome from the three distinct populations allows to cross validate and corroborate modelling insights. Our results show a previously under-recognized positive association between larger ICaL and DAD propensity; in that, increasing the conductance (GCaL) of L-type Ca2+ channels (LTCCs) or the PKA- or CaMKII-dependent phosphorylation levels (PKA-LTCC and CAMKII-LTCC) augments DAD probability, suggesting that augmenting ICaL can substantially contribute to spontaneous Ca2+ releases (SCRs) and thus to DADs and tAPs, by increasing the intracellular Ca2+ loading during the pre-conditioning pacing beats. Notably, when removing the cells displaying tAPs from the analysis, GCaL remained the parameter that most positively correlates with the presence of sub-threshold DADs (Figure 3C), confirming the notion that increasing ICaL contributes to sub-threshold DAD generation. Also, increasing sarcoplasmic reticulum Ca2+ ATPase (SERCA) and INaL activity by elevating either velocity/conductance (VSERCA or GNaL) or the associated regulatory phosphorylations [PKA-phospholamban (PLB) that enhances SERCA Ca2+ uptake and CaMKII-INa] promotes DADs. Conversely, increasing INaK [via increasing VNaK or PKA-phospholemman (PLM) which enhances INaK], INCX, or atrial-predominant IKur (GKur or PKA-IKur) attenuates DAD susceptibility via directly or indirectly reducing intracellular Ca2+. Consistently, elevating the expression of PP1 that targets IKur channels (PP1-IKur) increases DAD susceptibility. Finally, Population-3 uncovered the roles of the signalling cascade intermediates in producing cellular DADs. Both AC and β1AR expressions (thus PKA activity) are positively linked to DAD probability, whereas increasing the levels of phosphodiesterases (PDE3 and PDE4, which decrease active cAMP), protein phosphatases targeting LTCC (PP1-LTCC and PP2A-LTCC), or applying a PKA inhibitor peptide (PKI) all suppress DAD propensity. Collectively, these simulations provide novel and coherent mechanistic insights into the precise roles and the specific contributions of key ionic processes and upstream signalling systems to the propensity of human atrial cardiomyocytes to pro-arrhythmic triggered activity.
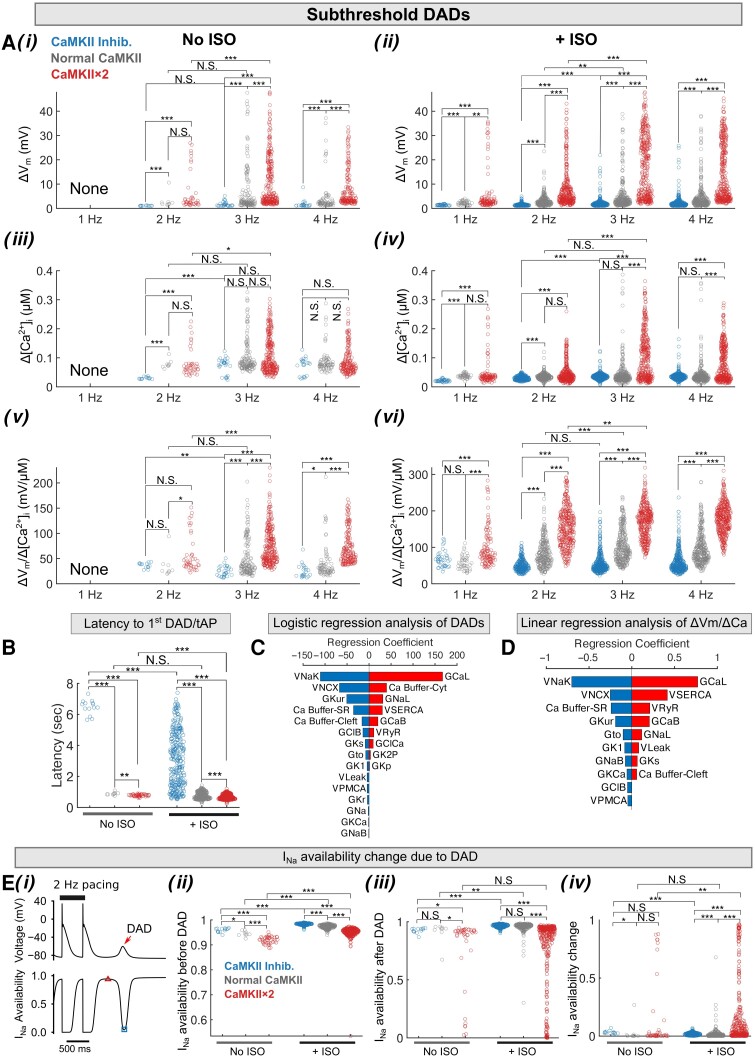
Figure 3.
Populations of models reveal rate dependence of sub-threshold DADs in human atrial cardiomyocytes following a pace-pause protocol. (A) Rate-dependent characterization of sub-threshold DAD: (i and ii) amplitudes of Vm instability (ΔVm) and (iii and iv) associated [Ca2+]i (Δ[Ca2+]i), and (v and vi) Vm to [Ca2+]i coupling (ΔVm/Δ[Ca2+]i); the left and right columns display data for no ISO conditions and after ISO treatment, respectively. (B) Latency to developing first sub-threshold DAD or tAP after cessation of 2-Hz stimulation. (C) Logistic regression analysis of sub-threshold DAD incidence in a subpopulation of Population-1 comprising model variants displaying only sub-threshold DADs and those showing no membrane instability for CaMKII × 2 + ISO application. (D) Linear regression analysis reveals the influence of subcellular parameters on the ΔVm/Δ[Ca2+]i of sub-threshold DADs. (E) CaMKII activation reduces INa availability following a sub-threshold DAD. (i) Illustration of tAPs and sub-threshold DADs after 2 Hz pacing, and the accompanying INa availability. The maximum INa availability before a sub-threshold DAD is indicated with a triangle, and its minimum after a sub-threshold DAD is marked with a square. (ii) Comparison of maximum INa availability before sub-threshold DADs. (iii) Comparison of minimum INa availability after sub-threshold DADs. (iv) The INa availability change due to sub-threshold DAD (computed as the difference between maximum and minimum availability). Statistical analysis was performed using Kruskal–Wallis test followed by planned comparisons with Wilcoxon rank sum test and Bonferroni correction. ***P < 0.001; **P < 0.01; *P < 0.05; N.S., not significant.
4. PKA and CaMKII impact the pacing rate-dependence of the incidence and amplitude of SCR events and the coupling with the resulting DADs and tAPs
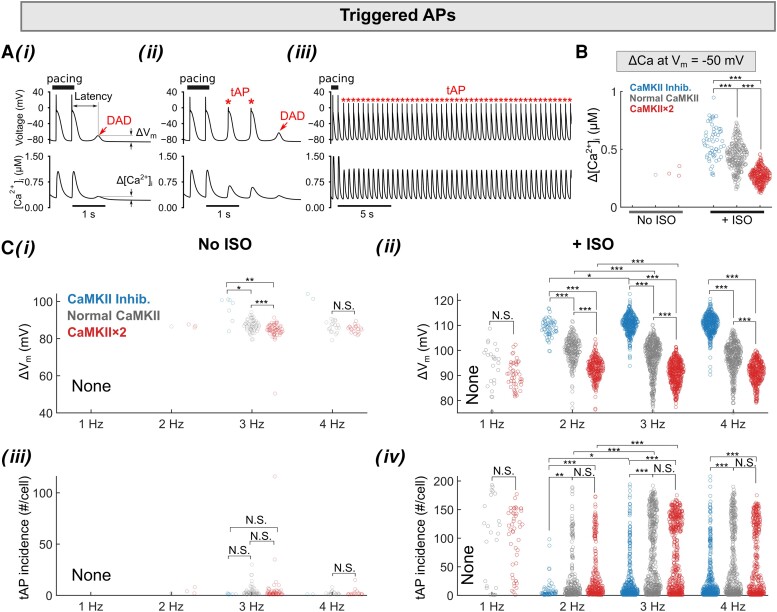
Due to corresponding elevation of [Ca2+]i (see Supplementary material online, Figure S11), CaMKII activity is a key mediator of cardiomyocyte responses to changes in pacing frequency. Our model captures the dynamic changes of CaMKII activity during the cardiac cycle at varying pacing rates (see Supplementary material online, Figure S11) and recapitulates both the beat-to-beat changes in CaMKII activation and target phosphorylation, as well as their frequency dependence (see Supplementary material online, Figure S11). We further examined the frequency dependence of triggered activity in our simulations with Population-1 (Figures 2 and 3), which displays the larger phenotypic variability when simulating the same range of perturbations in model parameters (i.e. larger standard deviation in AP and CaT parameters as shown in Supplementary material online, Figure S9). Depending on the amplitude of membrane depolarization, triggered activity manifests as sub-threshold DADs or tAPs (Figure 2Ai–iii). Examining the underlying ryanodine receptor 2 (RyR2), Na+/Ca2+ exchanger (NCX), and other ion channel activities confirms that these triggered activity events are initiated by SCRs that potentiate INCX and cause subsequent membrane depolarization (see Supplementary material online, Figure S12). Supplementary material online, Figure S13 illustrates the relationship between the amplitude of SCR (Δ[Ca2+]i) and the resulting DAD or tAP: the ΔVm increases with increasing Δ[Ca2+]i until it is large enough to trigger a tAP; after this threshold, the tAP amplitude barely increases even though Δ[Ca2+]i continues to grow. Both PKA and CaMKII negatively shift this relationship, whereby smaller Δ[Ca2+]i causes the greater depolarization increasing Vm-Ca2+ coupling gain (ΔVm/Δ[Ca2+]i). We further assessed the frequency dependence of the amplitude and the incidence of tAPs (Figure 2B and C), SCRs, DADs, and ΔVm/Δ[Ca2+]i for sub-threshold DADs for each atrial cardiomyocyte (Figure 3A and B) and determined the impact of PKA and CaMKII on these parameters. We found that ISO treatment and increasing pacing frequency markedly enhanced the propensity for tAPs, by increasing both the number of cells developing tAPs (Figure 2B and Ci–ii) and the incidence per cell (Figure 2Ciii and iv). The burden of tAPs was markedly reduced with CaMKII inhibition (Figure 2Ciii and iv), although the amplitude was increased (Figure 2Cii) due to CaMKII effects to reduce INa availability. Furthermore, we found that CaMKII reduced the threshold Ca2+ amplitude that triggers an AP (Figure 2B), measured as the [Ca2+]i value at Vm = −50 mV.
Figure 2.
Populations of models reveal rate dependence of tAPs in human atrial cardiomyocytes following a pace-pause protocol. (A) Illustration of three types of membrane Vm instabilities following a pace-pause protocol: (i) sub-threshold DAD only, (ii) both sub-threshold DAD and tAPs, and (iii) long-lasting tAPs. (B) Threshold Ca2+ amplitude causing tAPs is measured as the [Ca2+]i when Vm depolarizes to −50 mV. (C) Rate-dependent characterization of tAPs without (i and iii) and with (ii and iv) ISO treatment: (i and ii) mean voltage amplitude and (iii and iv) number of tAPs for each cardiomyocyte that developed tAPs. Statistical analysis was performed using Kruskal–Wallis test followed by planned comparisons with Wilcoxon rank sum test and Bonferroni correction. ***P < 0.001; **P < 0.01; *P < 0.05; N.S., not significant.
Our data demonstrate that pacing frequency, ISO administration, and CaMKII over-expression increase the incidence and amplitude of sub-threshold DADs (Figure 3Ai and ii) and SCRs (Figure 3Aiii and iv) and promote ΔVm/Δ[Ca2+]i (Figure 3Av–vi). Notably, CaMKII inhibition increases the latency to first DAD/tAP (Figure 3C) and decreases the amplitude of the SCR and DAD, and ΔVm/Δ[Ca2+]i, either in the absence or presence of ISO simulation (Figure 3A). Linear regression analysis revealed that the parameters that most strongly correlated with changes in ΔVm/Δ[Ca2+]i (Figure 3D) are the same that allow distinguishing between cells displaying DADs and those without DADs when applying logistic regression (Figure 3C). Interestingly, CaMKII hyper-activation also reduces INa availability following a sub-threshold DAD (Figure 3E), suggesting a critical role for sub-threshold DADs for increasing dispersion of refractoriness by impacting INa availability.
4.1. PKA and CaMKII synergistically promote DADs and triggered action potentials in electrically coupled heterogeneous tissue
Although we could establish a synergistic interplay between PKA and CaMKII that promotes triggered activity at the cellular level, it remains unclear whether triggered activity at the single-cell level persists in electrically coupled tissue, where the electrical coupling could dampen the triggered activity due to the current sink by surrounding cardiomyocytes.79,82,83 Indeed, to facilitate in-tissue tAP propagation, the source current density of depolarizing myocytes must be sufficiently strong to overcome the sink by the surrounding repolarized tissue.82,83 Besides, CaMKII may further modify the in-tissue arrhythmogenesis through a direct modulation of gap junctions52 and function of other ion fluxes that impact the source–sink relationship. Finally, the arrhythmia propensity at the tissue level may be affected by the degree of electrophysiological heterogeneity, which is a hallmark of the atria and critically governs arrhythmia dynamics.
To assess the contributions of PKA and CaMKII to triggered activity in tissue, we built an electrically heterogeneous and coupled tissue model (Figure 4) by creating a ‘grid-like’ mosaic tissue pattern and mapping the populations of our integrate models (Population-1) to the tissue, as detailed in Supplementary material online, Supplementary Methods and Figure S23. The two-dimensional (2D) atrial tissue was paced following a 2 Hz pacing (five beats) and pause protocol, allowing for characterizing AP conduction properties and membrane voltage instability events. Figure 4A and Supplementary material online, Movie S1 illustrate the tissue voltage map and extracted single cell APs following the last pacing stimulation. The spatial distributions and density of DADs in tissue are quantified in Figure 4B. Our simulations show normal AP wave propagation and repolarization in the control conditions + normal CaMKII (Figure 4Ai and Bi left column). However, DADs emerged across the tissue after ISO application (Figure 4Aii and Bi 2nd left column) and were largely suppressed with CaMKII inhibition (Figure 4Aiii and Bi 2nd right column). Remarkably, with increased CaMKII expression (two-fold, mimicking the CaMKII up-regulation in chronic AF patients12,18,20,22,84), ISO exacerbated the post-repolarization membrane instabilities (Figure 4Bi right column), which degenerated into tAPs propagating throughout the tissue (Figure 4Aiv). These tAPs originated from the edge/border of the tissue, where the electrical coupling was much weaker. Similar observations were reported in a previous study of engineered human heart tissue from induced pluripotent stem cell-derived ventricular cardiomyocytes.79 Quantified densities of DAD incidence are again consistent with a synergistic interplay between PKA and CaMKII in promoting DADs in the electrically coupled tissue (Figure 4Bii). Furthermore, PKA activation increased the conduction velocity (CV) of AP propagation in tissue, in agreement with reports from literature.85 Increasing CaMKII slowed CV with or without PKA activation, whereas the opposite effects were observed following CaMKII inhibition (see Supplementary material online, Figure S14). These results suggest that both PKA and CaMKII activation promote the propensity to develop transmembrane potential instabilities and tAPs in tissue. In addition, the CaMKII-dependent slowing of CV may create a substrate for AF-maintaining re-entry, another pivotal arrhythmic mechanism, by causing a conduction block and by reducing the wavelength of tissue electrical excitation.
Figure 4.
Effects of PKA and CaMKII activation on the membrane instabilities in simulated 2D atrial tissue. The 2D tissue slab was paced from the left side [site width indicated with a bar in top left of panel (i)] using a 2 Hz pacing (five beats) and pause protocol. The total simulated time was 10 s. (A, left) Time-stamped snapshots of tissue cell membrane voltage map featuring membrane voltage changes following the last pacing at t = 2000 ms. (A, right) Time courses of single-cell AP extracted from the tissue. The cell location is indicated in (A) (i), left. Panels (i–iv) illustrate (i) normal CaMKII without ISO, (ii) normal CaMKII with ISO, (iii) CaMKII inhibition with ISO, and (iv) two-fold CaMKII expression with ISO. Triggered AP initiation is indicated by arrows. (B) (i) Spatial distribution and (ii) density of DAD incidences in the tissue slab following the cessation of the pacing protocol. (C) (i) Simulated electrograms (EGMs) from the tissue simulations at reduced gap junction conductance (scaled to 75%, D × 75%) for the normal vs. CaMKII × 2 with ISO application. (ii) Effects of CaMKII inhibition or two-fold CaMKII expression on the number of tAP propagation and cycle length (CL) for normal and reduced gap junction conductances (D scaled from 100 to 25%).
Since triggered activity is often associated with structural remodelling, we assessed the PKA- and CaMKII-dependent propensity of tissue for tAPs with various degree of structural remodelling (e.g. as with atrial enlargement and fibrosis) and gap junction abnormalities3 (Figure 4C). Specifically, we varied the cell-to-cell electrical coupling strength between 100 and 25% and quantified the number of tAPs in tissue by examining the number of triggered activations from computed electrograms (EGMs) (Figure 4Ci). In the absence of ISO, tAPs were not detected in any of the CaMKII expression settings, even when considering the most severe electrical decoupling (to 25% of the basal value) (see Supplementary material online, Figure S15). Following application of ISO, simulations with normal CaMKII displayed a tissue coupling disruption-dependent increase in the incidence of tAPs and progressively shortened cycle length (CL) of the spontaneous activity. The tAP number was further increased with abbreviated CLs for CaMKII × 2 vs. the normal CaMKII groups (Figure 4C). Interestingly, this increase is more evident with scaling of tissue conductivity between 100 and 75% (Figure 4Cii), suggesting that the CaMKII-dependent propensity to triggered activity persists even with normal tissue conductivity. Thus, whereas in regions with preserved tissue conductivity, only the pathologically up-regulated CaMKII, as seen in chronic AF patients,12,18,20,22,84 was associated with tAP generation after ISO, in regions with strongly reduced tissue conductivity, which mimics AF-related structural remodelling, ISO produced tAP in the presence of physiological CaMKII levels. Overall, no tAPs were detected with ISO application after CaMKII inhibition in either group, suggesting that CaMKII is required and indispensable for PKA-induced triggered activity in tissue, as noted in a previous experimental study in human-engineered ventricular tissue.79 Interestingly, acute elimination of CaMKII actions on RyR2 for CaMII × 2 + ISO could also abolish tAPs while reducing DAD occurrence in tissue (see Supplementary material online, Figure S16), confirming a crucial contribution of CaMKII-dependent regulation of RyR2 to triggered activity in experimental AF paradigms and AF patients.14,22,86–88
4.2. CaMKII increases propensity to triggered activity in tissue by acting on the determinants of the source–sink relationship
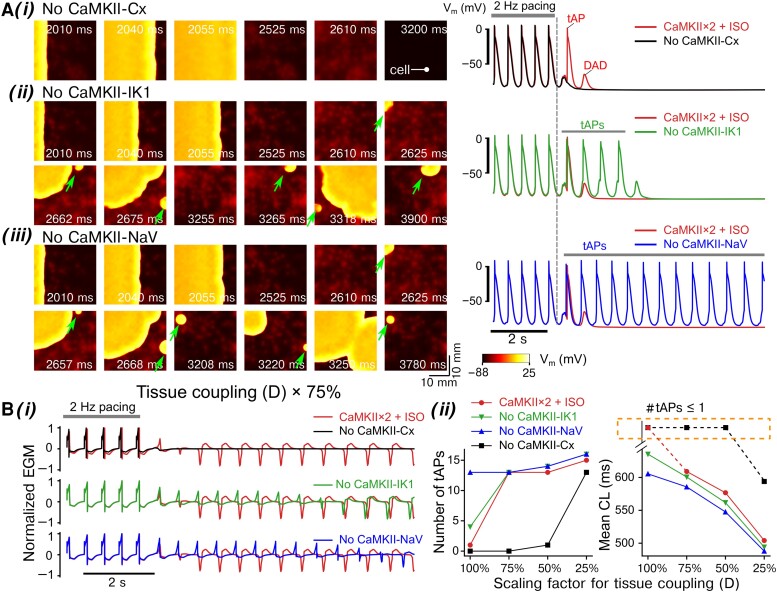
Although our simulations demonstrate a clear requirement of CaMKII for ISO-induced tAP in tissue, it is unknown whether and how CaMKII modulate the determinants of electrotonic load, thereby causing triggered activity. Indeed, previous experimental studies report that Ca2+-CaM reduces the connexin (Cx) conductivity,53 while inhibiting CaMKII increases tissue CV.52 Also, CaMKII causes a hyperpolarizing shift in the voltage-dependence of INa channel availability,89 thereby decreasing INa channel availability and limiting cellular excitability.90,91 Furthermore, the IK1 current is augmented by CaMKII,92 potentially elevating the sink of the tissue to be depolarized. We applied our model to dissect the mechanisms by which the CaMKII-dependent modifications to tissue parameters (namely, Cx function, IK1 activity, and voltage-dependence of INa availability, Supplementary material online, Figure S17 and Table S4) impact the propensity to triggered activity in tissue. Of note, these parameters are also important determinants of re-entry.3,10,93 We repeated the simulations of ISO + CaMKII × 2 group, but with removal of CaMKII-dependent modulations on each target individually (i.e. no CaMKII-Cx, no CaMKII-NaV, or no CaMKII-IK1, respectively). Simulated time courses of tissue voltage maps and extracted single-cell APs are shown in Figure 5A and Supplementary material online, Movie S2. Interestingly, excluding the CaMKII-dependent effect on Cx prevented the degeneration of DADs to propagating tAPs (Figure 5Ai), while increasing tissue CV (see Supplementary material online, Figure S18). This result suggests that CaMKII-dependent reduction of Cx conductance promotes the propensity to tAPs in tissue. In contrast, removing the CaMKII-dependent modulation on IK1 strongly increased the incidence of tAP (Figure 5Aii). The number of tAPs was highest when the CaMKII effect on INa availability was excluded: these tAPs originated from two distinct ectopic sites and sustained throughout the duration of simulation (Figure 5Aiii). Interestingly, tissue CV was not substantially modified in either of the last two groups, though in general a slight increase was noted (see Supplementary material online, Figure S18).
Figure 5.
Dissecting the roles of CaMKII-dependent modulations of connexins (CaMKII-Cx), IK1 (CaMKII-IK1), and Na+ channel availability (CaMKII-NaV) in inducing tAPs in tissue. The 2D tissue slab was paced from the left side [site width indicated with a bar in top left of panel (i)] using a 2 Hz pacing (five beats) and pause protocol. The total simulated time was 10 s. (A, left) Time-stamped snapshots of tissue cell membrane voltage map featuring membrane voltage changes following the last pacing at t = 2000 ms. (A, right) Time courses of single cell AP extracted from the tissue. The location of the cell is marked in (A) (i), left. (i–iii) illustrate simulations after ISO application with two-fold CaMKII expression but removing CaMKII-dependent modulations of (i) gap junctions (No CAMKII-Cx), (ii) IK1 (No CaMKII-IK1), and (iii) Na+ channel availability (No CaMKII-NaV). The 2D tissue slab was paced from the left side using a 2 Hz pacing (five beats) and pause protocol. Triggered AP initiation is indicated by arrows. (B) (i) Simulated EGMs from the tissue simulations at reduced gap junction conductance (scaled to 75%, D × 75%) showing effects of removing the CaMKII-dependent modulations on gap junctions (Cx), IK1, and Na+ channel availability. (ii) Number of tAP propagation and CL for normal and reduced tissue conductivities (D scaled from 100 to 25%).
We finally determined the contribution of tissue coupling to CaMKII- and PKA-dependent pro-arrhythmia (Figure 5B). Simulated tissue EGMs (Figure 5Bi) were computed to quantify the number and CL of the tAP (Figure 5Bii). Remarkably, the tAP-suppressing effect of excluding CaMKII-Cx was preserved for scaling of D ≥ 50%, suggesting a major contribution of CaMKII-Cx effects to the generation of tAPs in the absence of severe tissue uncoupling. Conversely, removing CaMKII-IK1 or CaMKII-NaV shortened the tAP CLs and increased the number of tAPs. These effects were most pronounced with normal tissue coupling, suggesting a protective role of CaMKII-IK1 and CaMKII-NaV against tAPs, particularly in the absence of tissue conductance disturbances. Collectively, our models provide new insights into the key roles and precise contributions of each CaMKII-dependent tissue parameter to arrhythmia propensity and highlight a critical role for CaMKII modulation of gap junctions in promoting tAPs in tissue.
4.3. PKA and CaMKII synergistically promote DADs and tAPs in PV-like myocytes and tissue
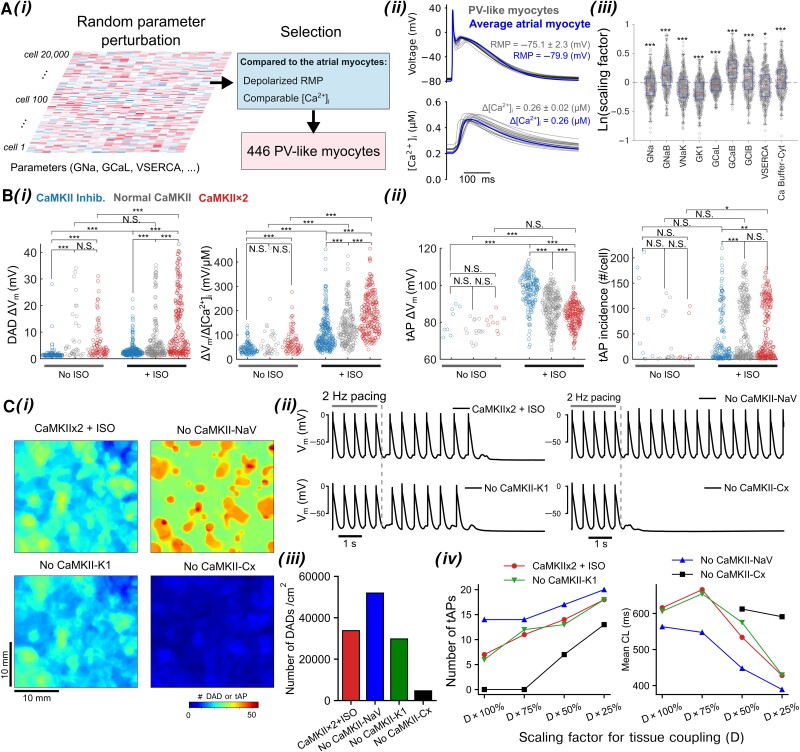
Triggered activity often occurs at the PV regions, where the PV cardiomyocytes have depolarized resting membrane potential (RMP)94 than the atrial cardiomyocytes due to a lower IK1.94,95 Given the interesting interactions between source and sink giving rise to triggered activity emerged at the tissue level, we investigated whether and how the source–sink balance are altered by PKA and CaMKII signalling in PV myocytes. We built a large (20 000) population of atrial cell models and selected a PV-like myocyte population as those exhibiting depolarized RMP94 and reduced upstroke velocity,95 but comparable [Ca2+]i96 with respect to the average atrial model (see Supplementary material online, Supplementary Methods; Figure 6Ai and ii). Analysing the parameters of PV-like population revealed that multiple subcellular processes significantly differed between the PV-like and the atrial cells (Figure 6Aiii), including lower IK1 and ICaL in the PV-like cells that have been documented in previous experimental studies.94–96 We characterized triggered activity in the PV-like population following 2-Hz pacing-pause protocol and found that the actions of PKA and CaMKII in the PV-like myocytes are consistent with those in atrial cells: both PKA and CaMKII activation augmented DAD amplitude and ΔVm/Δ[Ca2+]i (Figure 6Bi) while PKA activation also increased the number of cells exhibiting tAPs, whereas CaMKII inhibition reduced the number of tAPs in each PV-like myocyte (Figure 6Bii).
Figure 6.
Populations of PV-like myocytes and tissues demonstrate critical roles of PKA and CaMKII signals in promoting Vm and Ca2+ instabilities in PVs. (A) (i) Illustration of generating PV-like cardiomyocytes models by introducing random parameter perturbation to the average atrial cardiomyocyte model; model calibration selected PV-like cardiomyocytes as having depolarized RMP compared to the average LA cardiomyocytes while the [Ca2+]i being comparable. (A) (ii) Superimposed AP and [Ca2+]i traces of PV-like cardiomyocyte population and the average LA cardiomyocyte model paced at 1 Hz. (A) (iii) Identification of subcellular parameters of the PV-like cardiomyocyte population that are significantly different from the average LA cardiomyocyte. Note that the y-axis plots the scaling factors for those subcellular parameters in logarithm, and the scaling factors of the average atrial cardiomyocyte are thus zero and indicated with a dashed line. Statistical test was performed using one-sample t-test with Bonferroni correction. (B) Characterization of (i, left) Vm amplitude and (i, right) ΔVm/Δ[Ca2+]i coupling strength for sub-threshold DADs, and (ii, left) mean amplitude and (ii, right) number of incidence for tAPs in each PV-like cardiomyocyte following a 2-Hz pacing-pause protocol. Statistical analysis was performed using Kruskal–Wallis test followed by planned comparisons with Wilcoxon rank sum test and Bonferroni correction. (C) Tissue simulations uncover precise contributions of CaMKII-dependent modulations of INa availability (CaMKII-NaV), IK1 activity (CaMKII-K1), and connexins (CaMKII-Cx). (i) Spatial distribution of sub-threshold DADs and tAPs in simulated tissue following 2 Hz pacing-pause protocol. (ii) APs of cardiomyocytes extracted from the tissue. (iii) Density of DAD and tAP incidence in tissue. (iv, left) incidence number and (iv, right) mean CL of tAPs in tissue with respect to gradual reduction of tissue coupling. ***P < 0.001; **P < 0.01; *P < 0.05; N.S., not significant.
Lastly, we determined the contribution of CaMKII-dependent modulations on INa, IK1, and Cx to propensity to triggered activity in a heterogeneous PV-like tissue (Figure 6Ci–iv). As seen in atrial tissue simulations, removing CaMKII actions on INa substantially increased the incidence of triggered activity, whereas excluding CaMKII modulation of Cx strongly decreased the propensity to triggered activity; these effects persist over a wide range of tissue conductivity (Figure 6Civ). However, the incidence of triggered activity remained unaffected when removing CaMKII effects on IK1, due to the lower IK1 amplitude in the PV-like cells. Of note, our simulations revealed substantially greater incidence of triggered activity both in PV-like tissue and single cells compared to the atrial populations, in agreement with clinical observations of AF-promoting triggered activity often arising from the PV region.97
5. Discussion
We have constructed a novel multi-scale human atrial model integrating electrophysiology and Ca2+ handling with PKA- and CaMKII-signalling pathways by assembling the currently available knowledge in the field. Through simulations of populations-of-models, we uncovered a synergistic interplay between PKA and CaMKII that promotes triggered activity at both the single cell and tissue scales in human atria. Logistic regression analyses dissected anti- from pro-arrhythmic ionic processes and signalling components, providing the foundation for informing novel therapeutic approaches against AF. Our simulations uncovered a previously unrecognized critical role of CaMKII in modifying the source–sink mismatch to favour pro-arrhythmic tAPs in atrial tissue. Overall, our study establishes key mechanistic roles of CaMKII in the generation of pro-arrhythmic triggered activity in the atria by acting on both subcellular and inter-cellular (tissue) determinants of atrial function.
5.1. Working model of interactive signalling that promotes triggered activity
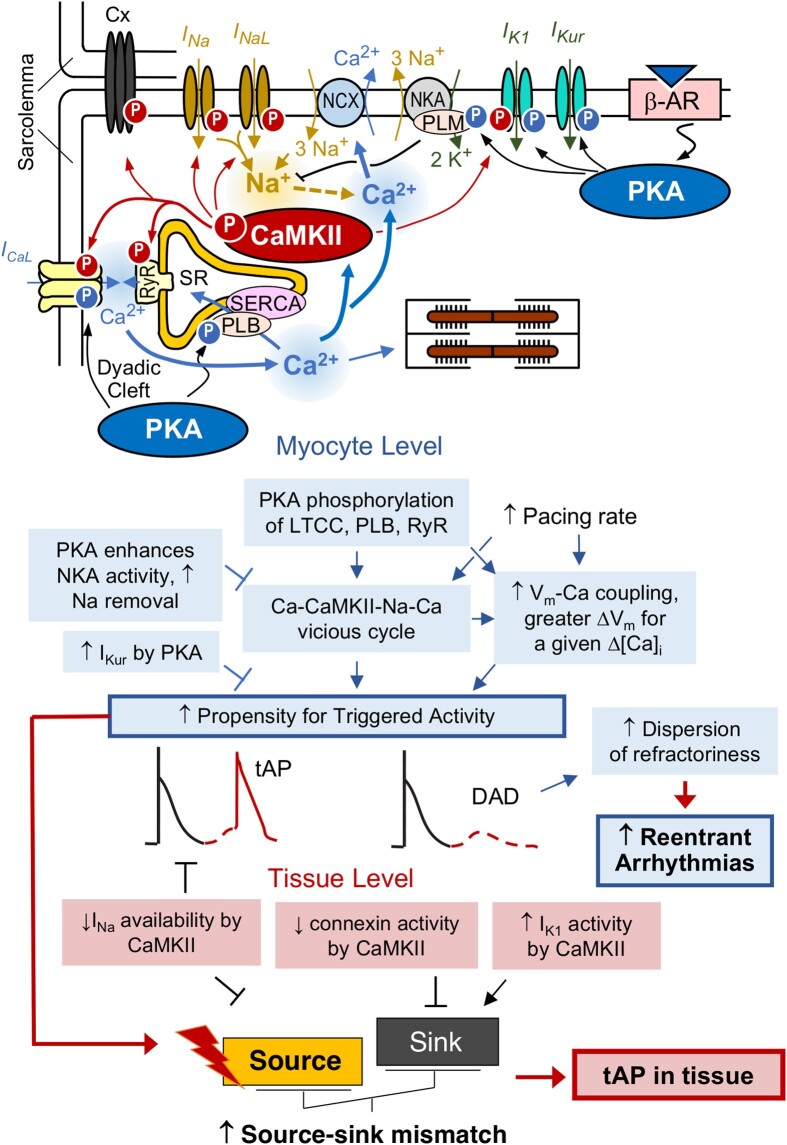
Our integrative computational model uncovered complex crosstalk patterns between PKA and CaMKII signalling in the promotion of DADs at both the cell and tissue levels of human atria (Figure 7). At the single-cell level, activated CaMKII and PKA both phosphorylate key Ca2+ handling proteins (PLB, RyR2, LTCC) involved in EC coupling, thereby increasing [Ca2+]i (Figure 7). The CaMKII-dependent augmentation of INaL function increases [Na+]i, promoting the outward shift of NCX that favours the elevation of [Ca2+]i (Figure 7). Concomitant PKA-dependent modifications of PLM enhance the activity of Na+/K+ ATPase (NKA), which decreases both [Na+]i and [Ca2+]i. Nevertheless, the CaMKII effects prevail, and the increased [Ca2+]i further enhances CaMKII activity, creating a vicious cycle of Ca2+/CaMKII/Na+/Ca2+ promoting DADs (Figure 7). Accordingly, previous experimental16,98 and computational99 studies revealed that CaMKII-dependent enhancement of INaL promoted Ca2+ overload and arrhythmogenesis. Likewise, increased Na+ influx per se promoted atrial arrhythmias in mice expressing human Nav1.5 with augmented persistent Na+ current.100 In addition, CaMKII activation could increase ΔVm/Δ[Ca2+]i coupling, further promoting triggered activity. This vicious arrhythmogenic cycle is amplified by PKA stimulation that targets the same Ca2+-handling proteins, thereby creating synergistic pro-arrhythmic effects. We propose that increased heart rates further drive this vicious cycle and ΔVm/Δ[Ca2+]i coupling. Furthermore, the factors promoting DADs at the single-cell level cause source–sink mismatch at the tissue level by directly augmenting the source, while CaMKII-dependent modifications to INa, connexins, and IK1 alter the source–sink relationship (Figure 7). For instance, CaMKII-dependent phosphorylation up-regulates IK1 and increases the current sink (counteracted by PKA-dependent IK1 decrease), while reducing the source by counteracting the depolarizing peak INa current. CaMKII activation also diminishes INa availability and thus cell excitability, thereby reducing the source. Both effects mitigate the mismatch between the source and sink. One the other hand, the reduced electrotonic coupling due to CaMKII phosphorylation of Cx substantially weakens the sink and synergizes with the increased current source (caused by the vicious cycle) to outweigh the CaMKII effects on IK1 and INa, thus exacerbating the source–sink mismatch that promotes triggered activity in tissue. Importantly, CaMKII inhibition abolishes triggered activity in both single cells and tissue, suggesting that CaMKII activity is required for human atrial triggered activity and arrhythmogenesis. Collectively, our simulations uncover a novel network mechanism of a synergistic pro-arrhythmic crosstalk between PKA and CaMKII and dissect mechanistically the specific roles and precise contributions of CaMKII targets at both the cell and tissue scales.
Figure 7.
Working model schematic illustrates the mechanisms underlying PKA and CaMKII activations promoting DADs and tAPs in both atrial myocytes and tissues.
5.2. Heterogeneous tissue simulations offer novel insights for tissue-level atrial arrhythmia
We constructed a heterogeneous 2D tissue to account for the hallmark electrophysiological heterogeneities in atrial tissue, while allowing randomly mapping our single-cell population of models onto the tissue. This approach allowed us to investigate whether triggered activity seen at the single-cell level persisted in tissue. Our results show that the increased DAD propensity at the single-cell level due to the synergistic PKA-CaMKII crosstalk further synergizes with CaMKII-dependent functional decoupling of cell–cell electrotonic communication to induce tAP in atrial tissue. Although our tissue models were constructed from single-cell model populations, in which a substantial fraction of cells display DADs, the tAPs in tissue often originated from a limited number of sites that are frequently close to the tissue border with weaker electrotonic coupling. Interestingly, analogously localized tAP sites were observed in engineered human ventricular tissue.79 Likewise, weakly coupled cardiomyocytes within the failing hearts can depolarize membrane voltage to trigger APs by overcoming a weakened current sink of surrounding tissue.101 Furthermore, decreasing the basal tissue coupling strength exacerbated the spontaneous triggered activity and created arrhythmogenic foci giving rise to a train of spontaneous APs that propagated throughout the tissue. Therefore, it is conceivable that in the atria, highly arrhythmogenic foci can be created by combining (i) cellular arrhythmogenic DADs, (ii) CaMKII-dependent functional electrotonic decoupling, and (iii) locally weakened electrical coupling due to fibrosis.
Our simulations uncover a protective role of the CaMKII actions on INa availability and IK1 activity. Indeed, removing CaMKII regulation on INa availability from our models resulted in substantially strengthened arrhythmogenic foci (Figure 5), without markedly modifying the CV of paced APs. These results can be explained by the timing of dynamic CaMKII activity during paced APs and Ca2+-driven tAPs: in the paced APs, a delay between AP upstroke and CaT prevented peaking of CaMKII regulation of INa during AP upstroke, whereas during the early phase of Ca2+-driven tAPs, strong CaMKII regulation of INa (by high [Ca2+]i) diminished cell excitability and the development of tAPs. The latter has additional implications on the pro-arrhythmic effects of sub-threshold DADs that failed to invoke tAPs. Sub-threshold DADs not only may promote tissue dispersion of cell excitability due to heterogeneous voltage-dependent INa availability,102 as shown in our analysis (Figure 3E) and working model (Figure 7), thereby causing conduction block that promotes r-eentry,44 but can also augment this functional excitability heterogeneity via spatial dispersion of Ca2+/CaMKII activities that modify INa, both creating vulnerable substrates for arrhythmia induction and maintenance. Thus, our simulations discovered novel insights into the potential effects of CaMKII on re-entrant arrhythmias. Likewise, CaMKII-dependent modulation of IK1 activity also produces protective effects against triggered activity (Figure 5). The increased IK1 upon CaMKII phosphorylation may help to counteract Ca2+ overload-induced depolarizing INCX to limit the arrhythmogenic propensity and thus reduce the current source, while it also may substantially increase the current sink of the surrounding cells in tissue, thereby contributing to alleviating the source–sink mismatch. However, the up-regulation of IK1 stabilizes rotors and may help to sustain AF-maintaining re-entry.103 Combined, our simulations establish complex patterns of CaMKII actions in triggering atrial arrhythmogenesis across spatial scales, which allow to dissect (mal)adaptive from adaptive remodelling components.
PV is a major source of triggered activity in AF,94 and thus, PV isolation is routinely performed in catheter ablation treatment of AF. Our simulation results underscore a prominent involvement of IK1 in shaping the higher propensity of PV to pro-arrhythmia. Consistent with clinical observations, our populations of PV-like cells and tissue display substantially increased incidence of triggered activity compared to atrial cardiomyocytes. This may be explained by lower IK1 amplitude in PV myocytes compared to atrial cardiomyocytes, which could augment ΔVm/Δ[Ca2+]i gain in each myocyte and decrease the electrical sink, thereby exacerbating the source–sink mismatch in tissue.
5.3. Integrative framework to define (mal)adaptive remodelling associated with AF
It is well established that AF is associated with extensive remodelling of ion channels and Ca2+ handling proteins including their regulations by upstream signalling.3,10,93,104–107 Discerning the adaptive changes from maladaptive remodelling may provide a better understanding of AF pathophysiology and inform favourable anti-AF targets, but such effort requires a systems framework integrating the mechanistic contributions of AF-associated remodelling processes. To this end, our simulations reveal relative influences of each protein and its phosphorylation on the propensity to arrhythmia, thus providing mechanistic insights into determining the adaptive or maladaptive nature of the remodelling processes caused by AF. For example, our results show that the propensity to DADs is most sensitive to an increase in the maximum conductance of ICaL (GCaL) (Figure 1Di). Thus, ICaL might contribute to DADs particularly in patients with paroxysmal AF wherein ICaL is unchanged,108 whereas previously documented reductions of ICaL in chronic AF3,13,67,104,106,107,109 despite increased open probability110 could be an adaptive process to attenuate arrhythmia propensity, but at the expense of causing re-entry-promoting APD abbreviation.10,111 Similarly, PKA- and CaMKII-dependent phosphorylation of LTCCs are positively correlated with pro-arrhythmia by causing cellular Ca2+ overload and triggered activity (Figure 1Dii). Conversely, increased activity of protein phosphatase 2A and PDE8-mediated LTCC dephosphorylation107 that have been observed in atrial tissue from AF patients13,28 may constitute adaptive changes to reduce LTCC phosphorylation levels and the related cellular Ca2+ overload, but at the expense of causing re-entry-promoting APD abbreviation (Figure 1Diii). The augmented phosphorylation of PLB and RyR2 (e.g. due to abnormal local activity of PP1 within the multi-protein complexes) also promotes atrial arrhythmogenesis (Figure 1Dii), which is consistent with prior work showing that PP1 deficiency or stronger inhibition by endogenous inhibitor-1 within the RyR286,112 and PLB28,113 complexes is associated with cardiac arrhythmogenesis in experimental models and patients. Preventing RyR2 phosphorylation in our model suppresses DADs and tAPs (see Supplementary material online, Figure S16). Of note, augmented CaMKII-induced target phosphorylation causes source–sink mismatch, which can be partially corrected by CaMKII-dependent modifications of INa availability and IK1. Similarly, the enhanced IK1 in AF patients114–116 could also be interpreted as a compensatory mechanism against triggered activity, however, at the expense of shortening APD and promoting re-entry. Furthermore, increasing NCX expression diminishes propensity of DADs (Figure 1Di). Therefore, the hallmark increase of NCX1 documented in AF10,18,22,28,72 may constitute an adaptive process to counteract the arrhythmogenic Ca2+ overload. On the other hand, NCX up-regulation increases ΔVm/Δ[Ca2+]i in chronic AF, and this could promote DADs during increased diastolic SR Ca2+ leak. These two apparently opposing effects of increased NCX may be both operative in AF, with the net effect depending on the study conditions and disease stages. Overall, our analysis unravels a complex landscape of AF-induced (mal)adaptive changes that we speculate may have pro-arrhythmic or anti-arrhythmic consequences depending on the prevailing arrhythmogenic mechanism (triggered activity, re-entry, or both) in an individual patient. As such, effective anti-AF strategies should be selectively directed to correct maladaptively remodelled processes such as those we identified in present study, where effects of CaMKII and PKA could be either pro-arrhythmic or anti-arrhythmic. An attractive therapeutic strategy would therefore be the development of selective protein–protein interaction inhibitors, which could prevent CaMKII and PKA interactions with some of its targets while leaving other targets unaffected.
5.4. Limitations and future directions
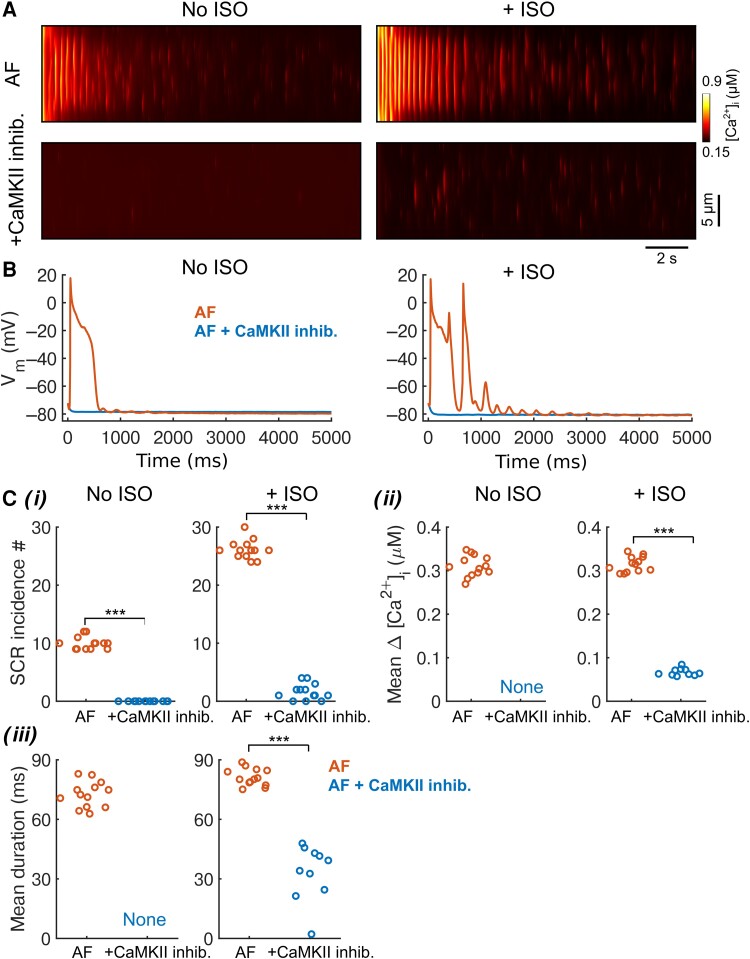
We acknowledge that our study has limitations that may be addressed in future investigations. First, β-adrenoceptor stimulation can activate CaMKII through βAR-cAMP-Epac–dependent117,118 or nitric oxide-dependent119 pathways that were not included in our modelling framework. The direct crosstalk between βAR signalling and CaMKII might further favour CaMKII activity during βAR simulation, thereby enhancing the synergistic crosstalk between PKA and CaMKII for promoting atrial arrhythmogenesis. Further, while the model captures the ability of CaMKII monomers to auto-phosphorylate neighbouring subunits thus prolonging the activated state of CaMKII, other post-translational modifications of CaMKII have been reported (oxidation,120O-GlcNAcylation,121 and S-nitrosylation122) and are not yet included in the model. The model could be further extended to include the role of inhibitor-1 as a PKA-dependent regulator of CaMKII phosphorylation,123,124 whereas in the current formulation, PKA activation of inhibitor-1 is linked to CaMKII through the increase in Thr-17 phosphorylation of PLB and contributes to the synergy of CaMKII and PKA signalling. While there are limited kinetic data available detailing the characteristics of CaMKII activation and target phosphorylation, the predicted outcomes are generally consistent with available experimental measurements and functional readouts. Second, we focused on studying the crosstalk effects by investigating propensity to triggered activity. However, the CaMKII-dependent increase in IK1, decrease in peak INa, and the Cx-mediated functional decoupling of tissue all could promote re-entry, another important arrhythmia mechanism.44,93,103 Indeed, the CaMKII-induced functional decoupling decreased CV in our simulations, which should reduce wavelength of excitation thereby promoting re-entry. However, we acknowledge that opposing effects of acute CaM/CaMKII inhibition on ventricular CV have been reported.52,53,125–127 Due to the extensive targets of CaMKII involved in determining CV, the exact action of CaMKII on Cx remains to be resolved. Nevertheless, our simulations show that CaMKII could induce sub-threshold DADs in tissue in the absence of its effect on Cx, and these sub-threshold DADs could create a substrate for re-entry by causing dispersion of excitability and conduction block. Of note, Cx can also be phosphorylated by other kinases including PKA and PKC, and the phosphorylation levels of Cx are high at basal conditions and are reduced with diseases including heart failure,128,129 whereas the exact effects of PKA on Cx are mixed from previous studies.130,131 Nevertheless, our additional simulations in which tissue connectivity was modestly (+10%) increased in the presence of ISO (see Supplementary material online, Figure S19) suggested that PKA effects on connexins only show minor impacts on the tAP properties in tissue simulations. Thus, our study provides novel mechanistic insights into PKA- and CaMKII-dependent arrhythmogenesis that should be experimentally demonstrated and validated in atrial tissue. Third, while our model incorporates detailed descriptions of protein phosphatase regulations for each substrate, it is important to acknowledge that the function of many of these phosphatase isoforms and targeting properties remains to be elucidated.132,133 The same applies to phosphodiesterase isoforms and regulations.107 Our model assumes that the biophysical and biochemical properties of phosphorylation regulations are shared among many substrates. New experimental insights on the substrate-specific phosphorylation regulations, as they emerge, may be included to update our model to tease out contribution of individual substrate-specific protein phosphorylation in arrhythmogenesis and implications for therapy. Fourth, our model is deterministic and lacks descriptions of spatial subcellular details of Ca2+ diffusion. Merging the spatially detailed stochastic models27,134,135 with the PKA and CaMKII signalling model presents several challenges and introduces uncertainties in model parameters, including spatial details of concentrations and activity of these kinases; in addition, to tease out the distinct effects of signalling on arrhythmogenesis, we did not explicitly include all AF-induced remodelling changes in our integrated signalling model. Nevertheless, we performed additional preliminary simulations using our recently developed 3D spatial model of human atrial cardiomyocytes incorporating stochastic subcellular Ca2+ signalling and AF-induced remodelling effects.134 We found that AF-induced remodelling caused substantially increased Ca2+ release events and associated INCX that overweighed IK1 (see Supplementary material online, Figure S20) leading to tAPs that are amplified by ISO treatment; CaMKII inhibition suppressed these events in quiescent cells (Figure 8A–C) and attenuated the diastolic SCRs during steady-state pacing (see Supplementary material online, Figure S21). These results are consistent throughout our study using distinct types of models. Fifth, we simulated a heterogeneous tissue with small clusters of randomly varied electrophysiological properties. While the presence of electrophysiological heterogeneities in the heart is well known, the spatial arrangement of these heterogeneities is poorly elucidated. Also, the spatial distribution of cardiac innervation and expression of adrenoceptors as well as CaMKII in the atria and their AF-related changes have not been fully understood. Although CaMKIIδ is considered the main cardiac isoform, CaMKIIγ contributes importantly to cardiac remodelling.136 Thus, future computational models should implement the contribution of different CaMKII isoforms. Our model framework can be readily modified to account for these critical spatial details, when available, to better understand the contribution of these heterogeneities in physiology and pathophysiology. Finally, as sex differences in cAMP/PKA pathway have been reported to contribute to sex differences in the Ca2+-handling properties of cardiomyocytes,137,138 our novel integrative modelling framework provides a unique platform for studying sex differences in the regulation of upstream signalling on the cardiac EC coupling, which is likely to contribute to mechanistic discovery of sex-dependent differences in arrhythmogenesis.
Figure 8.
Three-dimensional spatial model of non-stimulated human atrial cardiomyocytes shows that AF-induced remodelling and ISO treatment promote tAPs and Ca2+ release events, which are ameliorated by CaMKII inhibition. The spatial cell model was pre-conditioned with same initial conditions (i.e. same ion channel states, local Ca2+ concentrations in each compartment, etc.) before switching on AF remodelling effects, and adding ISO or CaMKII inhibition treatment effects. (A) Simulated transversal line-scan of [Ca2+]i in AF-remodelled human atrial cardiomyocytes showing effects of ISO treatment or CaMKII inhibition. (B) Membrane Vm of AF-remodelled cardiomyocytes and following treatments. (C) Characterization of SCR events in response to treatments measured with 13 transversal line scans spaced by 5.5 μm along the longitudinal axis. (i) Number of SCR incidence, (ii) mean [Ca2+]i amplitude of SCR, and (iii) mean duration of SCR. Two-sample Student’s t-test was applied to perform statistical test. ***P < 0.001.
We envision extending our integrative framework to incorporate emerging experimental insights of upstream regulation of cardiac EC coupling and to investigate a broad spectrum of arrhythmia mechanisms. As computational models are increasingly being applied in drug screening and therapeutic discovery,33,56,139,140 integrative models not only allow for studies under conditions where key signalling pathways are active or disrupted to faithfully represent arrhythmic hearts but also provide a framework to investigate non-ion channel anti-AF strategies (e.g. targeting upstream or downstream signalling). Thus, our integrative models are powerful and instrumental to assemble and reconcile existing knowledge into a coupled network, allowing for quantitatively dissecting the precise contributions of subcellular processes and their modifications by upstream regulatory pathways to initiation and maintenance of arrhythmia in the atria.
Supplementary Material
Contributor Information
Haibo Ni, Department of Pharmacology, University of California Davis, 451 Health Sciences Drive, Davis, CA 95616, USA.
Stefano Morotti, Department of Pharmacology, University of California Davis, 451 Health Sciences Drive, Davis, CA 95616, USA.
Xianwei Zhang, Department of Pharmacology, University of California Davis, 451 Health Sciences Drive, Davis, CA 95616, USA.
Dobromir Dobrev, Institute of Pharmacology, Faculty of Medicine, University Duisburg-Essen, Essen, Germany; Department of Medicine and Research Center, Montreal Heart Institute and Université de Montréal, Montréal, Canada; Department of Molecular Physiology and Biophysics, Baylor College of Medicine, Houston, TX, USA.
Eleonora Grandi, Department of Pharmacology, University of California Davis, 451 Health Sciences Drive, Davis, CA 95616, USA.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by American Heart Association 20POST35120462 (Postdoctoral Fellowship to H.N.) and 20PRE35120465 (Predoctoral Fellowship to X.Z.); National Heart, Lung, and Blood Institute NHLBI Grants R01HL131517 (E.G. and D.D.), R01HL141214 and P01HL141084 (E.G.), R01HL136389 (D.D.), R01HL089598 (D.D.), R01HL163277 (D.D.), R01HL160992 (D.D.), R00HL138160 (S.M.); NIH Stimulating Peripheral Activity to Relieve Conditions Grant 1OT2OD026580-01 (E.G.); University of California Davis School of Medicine Dean’s Fellow Award (E.G.); the European Union (large-scale integrative project MAESTRIA, No. 965286, D.D.); and Burroughs Wellcome Fund—Doris Duke Charitable Foundation ‘COVID-19 Fund to Retain Clinical Scientists’ Award (S.M.).
Data availability
The data underlying this article are available in the article and in its online supplementary material. Source codes, analysis scripts, and related parameter data are available for download at elegrandi.wixsite.com/grandilab/downloads and github.com/drgrandilab.
Translational perspective.
Despite significant advancement in our understanding of pathological mechanisms and alterations underlying atrial fibrillation (AF), a highly prevalent clinical arrhythmia causing substantial health and socio-economic burden, development of effective pharmacological therapeutics for AF remains an urgent unmet clinical need. We built a systems framework integrating key processes and their regulatory upstream signalling pathways that are involved in atrial electrophysiology and modified by AF. By simulating populations of single atrial cardiomyocyte models and heterogeneous tissues, our analysis demonstrated synergistic interactions between upstream signalling pathways that promote atrial arrhythmogenesis across spatial scales, added new insight into complex atrial arrhythmia mechanisms, and revealed adaptive and maladaptive alterations caused by AF, thus providing a powerful new tool for identifying innovative therapeutic approaches against AF.
References
- 1. Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation relationships among clinical features, epidemiology, and mechanisms. Circ Res 2014;114:1453–1468. [DOI] [PubMed] [Google Scholar]
- 2. Chung MK, Refaat M, Shen W-K, Kutyifa V, Cha Y-M, Di Biase L, Baranchuk A, Lampert R, Natale A, Fisher J, Lakkireddy DR. Atrial fibrillation. J Am Coll Cardiol 2020;75:1689–1713. [DOI] [PubMed] [Google Scholar]
- 3. Nattel S, Heijman J, Zhou L, Dobrev D. Molecular basis of atrial fibrillation pathophysiology and therapy: a translational perspective. Circ Res 2020;127:51–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heijman J, Guichard J-B, Dobrev D, Nattel S. Translational challenges in atrial fibrillation. Circ Res 2018;122:752–773. [DOI] [PubMed] [Google Scholar]
- 5. Michaud GF, Stevenson WG. Atrial fibrillation. N Engl J Med 2021;384:353–361. [DOI] [PubMed] [Google Scholar]
- 6. Staerk L, Sherer JA, Ko D, Benjamin EJ, Helm RH. Atrial fibrillation. Circ Res 2017;120:1501–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heijman J, Algalarrondo V, Voigt N, Melka J, Wehrens XHT, Dobrev D, Nattel S. The value of basic research insights into atrial fibrillation mechanisms as a guide to therapeutic innovation: a critical analysis. Cardiovasc Res 2016;109:467–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nattel S, Sager PT, Hüser J, Heijman J, Dobrev D. Why translation from basic discoveries to clinical applications is so difficult for atrial fibrillation and possible approaches to improving it. Cardiovasc Res 2021;117:1616–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nattel S, Harada M. Atrial remodeling and atrial fibrillation: recent advances and translational perspectives. J Am Coll Cardiol 2014;63(22):2335–2345. [DOI] [PubMed] [Google Scholar]
- 10. Heijman J, Voigt N, Nattel S, Dobrev D. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circ Res 2014;114:1483–1499. [DOI] [PubMed] [Google Scholar]
- 11. Bers DM, Grandi E. CaMKII regulation of cardiac ion channels. J Cardiovasc Pharmacol 2009;54:180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chelu MG, Sarma S, Sood S, Wang S, van Oort RJ, Skapura DG, Li N, Santonastasi M, Müller FU, Schmitz W, Schotten U, Anderson ME, Valderrábano M, Dobrev D, Wehrens XHT. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest 2009;119:1940–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Christ T, Boknik P, Wöhrl S, Wettwer E, Graf EM, Bosch RF, Knaut M, Schmitz W, Ravens U, Dobrev D. L-type Ca2+ current downregulation in chronic human atrial fibrillation is associated with increased activity of protein phosphatases. Circulation 2004;110:2651–2657. [DOI] [PubMed] [Google Scholar]
- 14. Heijman J, Muna AP, Veleva T, Molina CE, Sutanto H, Tekook M, Wang Q, Abu-Taha IH, Gorka M, Künzel S, El-Armouche A, Reichenspurner H, Kamler M, Nikolaev V, Ravens U, Li N, Nattel S, Wehrens XHT, Dobrev D. Atrial myocyte NLRP3/CaMKII nexus forms a substrate for postoperative atrial fibrillation. Circ Res 2020;127:1036–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heijman J, Voigt N, Wehrens XHT, Dobrev D. Calcium dysregulation in atrial fibrillation: the role of CaMKII. Front Pharmacol 2014;5:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lebek S, Pichler K, Reuthner K, Trum M, Tafelmeier M, Mustroph J, Camboni D, Rupprecht L, Schmid C, Maier LS, Arzt M, Wagner S. Enhanced CaMKII-dependent late i na induces atrial pro-arrhythmic activity in patients with sleep-disordered breathing. Circ Res 2020;126(5):603–615. [DOI] [PubMed] [Google Scholar]
- 17. Li N, Chiang DY, Wang S, Wang Q, Sun L, Voigt N, Respress JL, Ather S, Skapura DG, Jordan VK, Horrigan FT, Schmitz W, Müller FU, Valderrabano M, Nattel S, Dobrev D, Wehrens XHT. Ryanodine receptor–mediated calcium leak drives progressive development of an atrial fibrillation substrate in a transgenic mouse model. Circulation 2014;129:1276–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Neef S, Dybkova N, Sossalla S, Ort KR, Fluschnik N, Neumann K, Seipelt R, Schöndube FA, Hasenfuss G, Maier LS. CaMKII-dependent diastolic SR Ca2+ leak and elevated diastolic Ca2+ levels in right atrial myocardium of patients with atrial fibrillation. Circ Res 2010;106:1134–1144. [DOI] [PubMed] [Google Scholar]
- 19. Reinhardt F, Beneke K, Pavlidou NG, Conradi L, Reichenspurner H, Hove-Madsen L, Molina CE. Abnormal calcium handling in atrial fibrillation is linked to changes in cyclic AMP dependent signaling. Cells 2021;10:3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tessier S, Karczewski P, Krause EG, Pansard Y, Acar C, Lang-Lazdunski M, Mercadier JJ, Hatem SN. Regulation of the transient outward K(+) current by Ca(2+)/calmodulin-dependent protein kinases II in human atrial myocytes. Circ Res 1999;85:810–819. [DOI] [PubMed] [Google Scholar]
- 21. Vest JA, Wehrens XHT, Reiken SR, Lehnart SE, Dobrev D, Chandra P, Danilo P, Ravens U, Rosen MR, Marks AR. Defective cardiac ryanodine receptor regulation during atrial fibrillation. Circulation 2005;111:2025–2032. [DOI] [PubMed] [Google Scholar]
- 22. Voigt N, Li N, Wang Q, Wang W, Trafford AW, Abu-Taha I, Sun Q, Wieland T, Ravens U, Nattel S, Wehrens XHT, Dobrev D. Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation 2012;125:2059–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wakili R, Yeh Y-H, Qi XY, Greiser M, Chartier D, Nishida K, Maguy A, Villeneuve L-R, Boknik P, Voigt N, Krysiak J, Kääb S, Ravens U, Linke WA, Stienen GJM, Shi Y, Tardif J-C, Schotten U, Dobrev D, Nattel S. Multiple potential molecular contributors to atrial hypocontractility caused by atrial tachycardia remodeling in dogs. Circ Arrhythm Electrophysiol 2010;3:530–541. [DOI] [PubMed] [Google Scholar]
- 24. Workman AJ. Cardiac adrenergic control and atrial fibrillation. Naunyn Schmiedebergs Arch Pharmacol 2010;381:235–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yeh Y-H, Wakili R, Qi X-Y, Chartier D, Boknik P, Kääb S, Ravens U, Coutu P, Dobrev D, Nattel S. Calcium-handling abnormalities underlying atrial arrhythmogenesis and contractile dysfunction in dogs with congestive heart failure. Circ Arrhythm Electrophysiol 2008;1:93–102. [DOI] [PubMed] [Google Scholar]
- 26. Yoo S, Aistrup G, Shiferaw Y, Ng J, Mohler PJ, Hund TJ, Waugh T, Browne S, Gussak G, Gilani M, Knight BP, Passman R, Goldberger JJ, Wasserstrom JA, Arora R. Oxidative stress creates a unique, CaMKII-mediated substrate for atrial fibrillation in heart failure. . JCI Insight 2018;3:e120728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tarifa C, Vallmitjana A, Jiménez-Sábado V, Marchena M, Llach A, Herraiz-Martínez A, Godoy-Marín H, Nolla-Colomer C, Ginel A, Viñolas X, Montiel J, Ciruela F, Echebarria B, Benítez R, Cinca J, Hove-Madsen L. The spatial distribution of calcium sparks determines their ability to induce afterdepolarizations in human atrial myocytes. JACC Basic Transl Sci 2022;8(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. El-Armouche A, Boknik P, Eschenhagen T, Carrier L, Knaut M, Ravens U, Dobrev D. Molecular determinants of altered Ca2+ handling in human chronic atrial fibrillation. Circulation 2006;114:670–680. [DOI] [PubMed] [Google Scholar]
- 29. Grandi E, Dobrev D. Non-ion channel therapeutics for heart failure and atrial fibrillation: are CaMKII inhibitors ready for clinical use? J Mol Cell Cardiol 2018;121:300–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grandi E, Maleckar MM. Anti-arrhythmic strategies for atrial fibrillation: the role of computational modeling in discovery, development, and optimization. Pharmacol Ther 2016;168:126–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Morotti S, Edwards AG, McCulloch AD, Bers DM, Grandi E. A novel computational model of mouse myocyte electrophysiology to assess the synergy between Na+ loading and CaMKII. J Physiol 2014;592:1181–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dobrev D, Wehrens XHT. Role of RyR2 phosphorylation in heart failure and arrhythmias. Circ Res 2014;114:1311–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Boyle PM, Zghaib T, Zahid S, Ali RL, Deng D, Franceschi WH, Hakim JB, Murphy MJ, Prakosa A, Zimmerman SL, Ashikaga H, Marine JE, Kolandaivelu A, Nazarian S, Spragg DD, Calkins H, Trayanova NA. Computationally guided personalized targeted ablation of persistent atrial fibrillation. Nat Biomed Eng 2019;3(11):870–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grandi E, Dobrev D, Heijman J. Computational modeling: what does it tell us about atrial fibrillation therapy? Int J Cardiol 2019;287:155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Heijman J, Sutanto H, Crijns HJGM, Nattel S, Trayanova NA. Computational models of atrial fibrillation: achievements, challenges, and perspectives for improving clinical care. Cardiovasc Res 2021;117:1682–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Heijman J, Erfanian Abdoust P, Voigt N, Nattel S, Dobrev D. Computational models of atrial cellular electrophysiology and calcium handling, and their role in atrial fibrillation. J Physiol 2016;594:537–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bartos DC, Morotti S, Ginsburg KS, Grandi E, Bers DM. Quantitative analysis of the Ca2+-dependent regulation of delayed rectifier K+ current IKs in rabbit ventricular myocytes. J Physiol 2017;595:2253–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hegyi B, Morotti S, Liu C, Ginsburg KS, Bossuyt J, Belardinelli L, Izu LT, Chen-Izu Y, Bányász T, Grandi E, Bers DM. Enhanced depolarization drive in failing rabbit ventricular myocytes—calcium-dependent and β-adrenergic effects on late sodium, L-type calcium, and sodium-calcium exchange currents. Circ Arrhythm Electrophysiol 2019;12:e007061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Morotti S, Liu C, Hegyi B, Ni H, Fogli Iseppe A, Wang L, Pritoni M, Ripplinger CM, Bers DM, Edwards AG, Grandi E. Quantitative cross-species translators of cardiac myocyte electrophysiology: model training, experimental validation, and applications. Sci Adv 2021;7:eabg0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Soltis AR, Saucerman JJ. Synergy between CaMKII substrates and β-adrenergic signaling in regulation of cardiac myocyte Ca2+ handling. Biophys J 2010;99:2038–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Goette A, Kalman JM, Aguinaga L, Akar J, Cabrera JA, Chen SA, Chugh SS, Corradi D, D’Avila A, Dobrev D, Fenelon G, Gonzalez M, Hatem SN, Helm R, Hindricks G, Ho SY, Hoit B, Jalife J, Kim Y-H, Lip GYH, Ma C-S, Marcus GM, Murray K, Nogami A, Sanders P, Uribe W, Van Wagoner DR, Nattel S. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Heart Rhythm 2017;14:e3–e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Richards MA, Clarke JD, Saravanan P, Voigt N, Dobrev D, Eisner DA, Trafford AW, Dibb KM. Transverse tubules are a common feature in large mammalian atrial myocytes including human. Am J Physiol Heart Circ Physiol 2011;301:H1996–H2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Walden AP, Dibb KM, Trafford AW. Differences in intracellular calcium homeostasis between atrial and ventricular myocytes. J Mol Cell Cardiol 2009;46:463–473. [DOI] [PubMed] [Google Scholar]
- 44. Landstrom AP, Dobrev D, Wehrens XHT. Calcium signaling and cardiac arrhythmias. Circ Res 2017;120:1969–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Benito B, Brugada R, Perich RM, Lizotte E, Cinca J, Mont L, Berruezo A, Tolosana JM, Freixa X, Brugada P, Brugada J. A mutation in the sodium channel is responsible for the association of long QT syndrome and familial atrial fibrillation. Heart Rhythm 2008;5:1434–1440. [DOI] [PubMed] [Google Scholar]
- 46. Grandi E, Pandit SV, Voigt N, Workman AJ, Dobrev D, Jalife J, Bers DM. Human atrial action potential and Ca2+ model: sinus rhythm and chronic atrial fibrillation. Circ Res 2011;109:1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Morotti S, McCulloch AD, Bers DM, Edwards AG, Grandi E. Atrial-selective targeting of arrhythmogenic phase-3 early afterdepolarizations in human myocytes. J Mol Cell Cardiol 2016;96:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Saucerman JJ, Brunton LL, Michailova AP, McCulloch AD. Modeling β-adrenergic control of cardiac myocyte contractility in silico. J Biol Chem 2003;278:47997–48003. [DOI] [PubMed] [Google Scholar]
- 49. Saucerman JJ, Bers DM. Calmodulin mediates differential sensitivity of CaMKII and calcineurin to local Ca2+ in cardiac myocytes. Biophys J 2008;95:4597–4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yang JH, Saucerman JJ. Phospholemman is a negative feed-forward regulator of Ca2+ in β-adrenergic signaling, accelerating β-adrenergic inotropy. J Mol Cell Cardiol 2012;52:1048–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Morotti S, Grandi E, Summa A, Ginsburg KS, Bers DM. Theoretical study of L-type Ca2+ current inactivation kinetics during action potential repolarization and early afterdepolarizations. J Physiol 2012;590:4465–4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Takanari H, Bourgonje VJA, Fontes MSC, Raaijmakers AJA, Driessen H, Jansen JA, van der Nagel R, Kok B, van Stuijvenberg L, Boulaksil M, Takemoto Y, Yamazaki M, Tsuji Y, Honjo H, Kamiya K, Kodama I, Anderson ME, van der Heyden MAG, van Rijen HVM, van Veen TAB, Vos MA. Calmodulin/CaMKII inhibition improves intercellular communication and impulse propagation in the heart and is antiarrhythmic under conditions when fibrosis is absent. Cardiovasc Res 2016;111:410–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Toyama J, Sugiura H, Kamiya K, Kodama I, Terasawa M, Hidaka H. Ca2+-calmodulin mediated modulation of the electrical coupling of ventricular myocytes isolated from guinea pig heart. J Mol Cell Cardiol 1994;26:1007–1015. [DOI] [PubMed] [Google Scholar]
- 54. Gong JQX, Susilo ME, Sher A, Musante CJ, Sobie EA. Quantitative analysis of variability in an integrated model of human ventricular electrophysiology and β-adrenergic signaling. J Mol Cell Cardiol 2020;143:96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Morotti S, Grandi E. Logistic regression analysis of populations of electrophysiological models to assess proarrythmic risk. MethodsX 2017;4:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ni H, Iseppe AF, Giles WR, Narayan SM, Zhang H, Edwards AG, Morotti S, Grandi E. Populations of in silico myocytes and tissues reveal synergy of multiatrial-predominant K+-current block in atrial fibrillation. Br J Pharmacol 2020;177:4497–4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ni H, Morotti S, Grandi E. A heart for diversity: simulating variability in cardiac arrhythmia research. Front Physiol 2018;9:958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sobie EA. Parameter sensitivity analysis in electrophysiological models using multivariable regression. Biophys J 2009;96:1264–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Heijman J, Zhou X, Morotti S, Molina CE, Abu-Taha IH, Tekook M, Jespersen T, Zhang Y, Dobrev S, Milting H, Gummert J, Karck M, Kamler M, El-Armouche A, Saljic A, Grandi E, Nattel S, Dobrev D. Enhanced Ca2+-dependent SK-channel gating and membrane trafficking in human atrial fibrillation. Circ Res 2023;132(9):e116–e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Moreno JD, Yang P-C, Bankston JR, Grandi E, Bers DM, Kass RS, Clancy CE. Ranolazine for congenital and acquired late INa-linked arrhythmias: in silico pharmacological screening. Circ Res 2013;113:e50–e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Colman MA, Aslanidi OV, Kharche S, Boyett MR, Garratt C, Hancox JC, Zhang H. Pro-arrhythmogenic effects of atrial fibrillation-induced electrical remodelling: insights from the three-dimensional virtual human atria. J Physiol 2013;591:4249–4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Courtemanche M, Ramirez RJ, Nattel S. Ionic mechanisms underlying human atrial action potential properties: insights from a mathematical model. Am J Physiol Heart Circ Physiol 1998;275:H301–H321. [DOI] [PubMed] [Google Scholar]
- 63. Nygren A, Fiset C, Firek L, Clark JW, Lindblad DS, Clark RB, Giles WR. Mathematical model of an adult human atrial cell the role of K+ currents in repolarization. Circ Res 1998;82:63–81. [DOI] [PubMed] [Google Scholar]
- 64. Wang Z, Fermini B, Nattel S. Delayed rectifier outward current and repolarization in human atrial myocytes. Circ Res 1993;73:276–285. [DOI] [PubMed] [Google Scholar]
- 65. Franz MR, Karasik PL, Li C, Moubarak J, Chavez M. Electrical remodeling of the human atrium: similar effects in patients with chronic atrial fibrillation and atrial flutter. J Am Coll Cardiol 1997;30:1785–1792. [DOI] [PubMed] [Google Scholar]
- 66. Bosch RF, Zeng X, Grammer JB, Popovic K, Mewis C, Kühlkamp V. Ionic mechanisms of electrical remodeling in human atrial fibrillation. Cardiovasc Res 1999;44:121–131. [DOI] [PubMed] [Google Scholar]
- 67. Wagoner DRV, Pond AL, Lamorgese M, Rossie SS, McCarthy PM, Nerbonne JM. Atrial L-type Ca2+ currents and human atrial fibrillation. Circ Res 1999;85:428–436. [DOI] [PubMed] [Google Scholar]
- 68. Skibsbye L, Poulet C, Diness JG, Bentzen BH, Yuan L, Kappert U, Matschke K, Wettwer E, Ravens U, Grunnet M, Christ T, Jespersen T. Small-conductance calcium-activated potassium (SK) channels contribute to action potential repolarization in human atria. Cardiovasc Res 2014;103:156–167. [DOI] [PubMed] [Google Scholar]
- 69. Wettwer E, Christ T, Endig S, Rozmaritsa N, Matschke K, Lynch JJ, Pourrier M, Gibson JK, Fedida D, Knaut M, Ravens U. The new antiarrhythmic drug vernakalant: ex vivo study of human atrial tissue from sinus rhythm and chronic atrial fibrillation. Cardiovasc Res 2013;98:145–154. [DOI] [PubMed] [Google Scholar]
- 70. Ford J, Milnes J, El Haou S, Wettwer E, Loose S, Matschke K, Tyl B, Round P, Ravens U. The positive frequency-dependent electrophysiological effects of the IKur inhibitor XEN-D0103 are desirable for the treatment of atrial fibrillation. Heart Rhythm 2016;13:555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Maier LS, Barckhausen P, Weisser J, Aleksic I, Baryalei M, Pieske B. Ca2+ handling in isolated human atrial myocardium. Am J Physiol-Heart Circ Physiol 2000;279:H952–H958. [DOI] [PubMed] [Google Scholar]
- 72. Schotten U, Greiser M, Benke D, Buerkel K, Ehrenteidt B, Stellbrink C, Vazquez-Jimenez JF, Schoendube F, Hanrath P, Allessie M. Atrial fibrillation-induced atrial contractile dysfunction: a tachycardiomyopathy of a different sort. Cardiovasc Res 2002;53:192–201. [DOI] [PubMed] [Google Scholar]
- 73. de la Fuente M G, Barana A, Gómez R, Amorós I, Dolz-Gaitón P, Sacristán S, Atienza F, Pita A, Pinto Á, Fernández-Avilés F, Caballero R, Tamargo J, Delpón E. Chronic atrial fibrillation up-regulates β1-adrenoceptors affecting repolarizing currents and action potential duration. Cardiovasc Res 2013;97:379–388. [DOI] [PubMed] [Google Scholar]
- 74. Li GR, Nattel S. Properties of human atrial ICa at physiological temperatures and relevance to action potential. Am J Physiol Heart Circ Physiol 1997;272:H227–H235. [DOI] [PubMed] [Google Scholar]
- 75. Golowasch J, Goldman MS, Abbott LF, Marder E. Failure of averaging in the construction of a conductance-based neuron model. J Neurophysiol 2002;87:1129–1131. [DOI] [PubMed] [Google Scholar]
- 76. Marder E. Variability, compensation, and modulation in neurons and circuits. Proc Natl Acad Sci 2011;108:15542–15548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Britton OJ, Bueno-Orovio A, Ammel KV, Lu HR, Towart R, Gallacher DJ, Rodriguez B. Experimentally calibrated population of models predicts and explains intersubject variability in cardiac cellular electrophysiology. Proc Natl Acad Sci 2013;110:E2098–E2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lebek S, Plößl A, Baier M, Mustroph J, Tarnowski D, Lücht CM, Schopka S, Flörchinger B, Schmid C, Zausig Y, Pagratis N, Marchand B, Koltun DO, Hung WK, Ahmadyar S, Belardinelli L, Maier LS, Wagner S. The novel CaMKII inhibitor GS-680 reduces diastolic SR Ca leak and prevents CaMKII-dependent pro-arrhythmic activity. J Mol Cell Cardiol 2018;118:159–168. [DOI] [PubMed] [Google Scholar]
- 79. Sung-Jin P, Donghui Z, Yan Q, Yifei L, Yong LK, Vassilios JB, Pengcheng Y, Shutao X, Sean LK, Xujie L, Fujian L, Francesco SP, Patrick HC, Judith G, Amy ER, Andre GK, Dominic JA, William TP, Kit PK. Insights into the pathogenesis of catecholaminergic polymorphic ventricular tachycardia from engineered human heart tissue. Circulation 2019;140:390–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Redpath CJ, Rankin AC, Kane KA, Workman AJ. Anti-adrenergic effects of endothelin on human atrial action potentials are potentially anti-arrhythmic. J Mol Cell Cardiol 2006;40:717–724. [DOI] [PubMed] [Google Scholar]
- 81. Kettlewell S, Burton FL, Smith GL, Workman AJ. Chronic myocardial infarction promotes atrial action potential alternans, afterdepolarizations, and fibrillation. Cardiovasc Res 2013;99:215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Campos FO, Shiferaw Y, Prassl AJ, Boyle PM, Vigmond EJ, Plank G. Stochastic spontaneous calcium release events trigger premature ventricular complexes by overcoming electrotonic load. Cardiovasc Res 2015;107:175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Xie Y, Sato D, Garfinkel A, Qu Z, Weiss JN. So little source, so much sink: requirements for afterdepolarizations to propagate in tissue. Biophys J 2010;99:1408–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Anil P, Adam GR, Xiaoqun G, Biyi C, Olha MK, Niels V, Stefan N, Thomas S, Zhan G, Elizabeth DL, Hrafnhildur S, Andrew CB, Na L, Ramzi NE-A, Baoli Y, Dominic SP, Robert MW, Xander HTW, Long-Sheng S, Dobromir D, Lars SM, Mark EA. Oxidized Ca2+/calmodulin-dependent protein kinase II triggers atrial fibrillation. Circulation 2013;128:1748–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Grandi E, Ripplinger CM. Antiarrhythmic mechanisms of beta blocker therapy. Pharmacol Res 2019;146:104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Chiang DY, Li N, Wang Q, Alsina KM, Quick AP, Reynolds JO, Wang G, Skapura D, Voigt N, Dobrev D, Wehrens XHT. Impaired local regulation of ryanodine receptor type 2 by protein phosphatase 1 promotes atrial fibrillation. Cardiovasc Res 2014;103:178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Dobrev D, Wehrens XHT. Calcium-mediated cellular triggered activity in atrial fibrillation. J Physiol 2017;595:4001–4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Li N, Wang T, Wang W, Cutler MJ, Wang Q, Voigt N, Rosenbaum DS, Dobrev D, Wehrens XHT. Inhibition of CaMKII phosphorylation of RyR2 prevents induction of atrial fibrillation in FKBP12.6 knockout mice. Circ Res 2012;110:465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wagner S, Dybkova N, Rasenack ECL, Jacobshagen C, Fabritz L, Kirchhof P, Maier SKG, Zhang T, Hasenfuss G, Brown JH, Bers DM, Maier LS. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J Clin Invest 2006;116:3127–3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Christensen MD, Dun W, Boyden PA, Anderson ME, Mohler PJ, Hund TJ. Oxidized calmodulin kinase II regulates conduction following myocardial infarction: a computational analysis. PLOS Comput Biol 2009;5:e1000583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Grandi E, Puglisi JL, Wagner S, Maier LS, Severi S, Bers DM. Simulation of ca-calmodulin-dependent protein kinase II on rabbit ventricular myocyte ion currents and action potentials. Biophys J 2007;93:3835–3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wagner S, Hacker E, Grandi E, Weber SL, Dybkova N, Sossalla S, Sowa T, Fabritz L, Kirchhof P, Bers DM, Maier LS. Ca/calmodulin kinase II differentially modulates potassium currents. Circ Arrhythm Electrophysiol 2009;2:285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation mechanisms and implications. Circ Arrhythm Electrophysiol 2008;1:62–73. [DOI] [PubMed] [Google Scholar]
- 94. Cha T-J, Ehrlich JR, Zhang L, Chartier D, Leung TK, Nattel S. Atrial tachycardia remodeling of pulmonary vein cardiomyocytes comparison with left atrium and potential relation to arrhythmogenesis. Circulation 2005;111:728–735. [DOI] [PubMed] [Google Scholar]
- 95. Ehrlich JR, Cha T-J, Zhang L, Chartier D, Melnyk P, Hohnloser SH, Nattel S. Cellular electrophysiology of canine pulmonary vein cardiomyocytes: action potential and ionic current properties. J Physiol 2003;551:801–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Coutu P, Chartier D, Nattel S. Comparison of Ca2+-handling properties of canine pulmonary vein and left atrial cardiomyocytes. Am J Physiol Heart Circ Physiol 2006;291:H2290–H2300. [DOI] [PubMed] [Google Scholar]
- 97. Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659–666. [DOI] [PubMed] [Google Scholar]
- 98. Fischer TH, Herting J, Mason FE, Hartmann N, Watanabe S, Nikolaev VO, Sprenger JU, Fan P, Yao L, Popov A-F, Danner BC, Schöndube F, Belardinelli L, Hasenfuss G, Maier LS, Sossalla S. Late INa increases diastolic SR-Ca2+-leak in atrial myocardium by activating PKA and CaMKII. Cardiovasc Res 2015;107:184–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Onal B, Gratz D, Hund TJ. Ca2+/calmodulin-dependent kinase II-dependent regulation of atrial myocyte late Na+ current, Ca2+ cycling, and excitability: a mathematical modeling study. Am J Physiol-Heart Circ Physiol 2017;313:H1227–H1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wan E, Abrams J, Weinberg RL, Katchman AN, Bayne J, Zakharov SI, Yang L, Morrow JP, Garan H, Marx SO. Aberrant sodium influx causes cardiomyopathy and atrial fibrillation in mice. J Clin Invest 2016;126:112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Lang D, Sato D, Jiang Y, Ginsburg KS, Ripplinger CM, Bers DM. Calcium-dependent arrhythmogenic foci created by weakly coupled myocytes in the failing heart. Circ Res 2017;121:1379–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Liu MB, Ko CY, Song Z, Garfinkel A, Weiss JN, Qu Z. A dynamical threshold for cardiac delayed afterdepolarization-mediated triggered activity. Biophys J 2016;111:2523–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Pandit SV, Berenfeld O, Anumonwo JMB, Zaritski RM, Kneller J, Nattel S, Jalife J. Ionic determinants of functional reentry in a 2-D model of human atrial cells during simulated chronic atrial fibrillation. Biophys J 2005;88:3806–3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Dobrev D, Ravens U. Remodeling of cardiomyocyte ion channels in human atrial fibrillation. Basic Res Cardiol 2003;98:137–148. [DOI] [PubMed] [Google Scholar]
- 105. Wakili R, Voigt N, Kääb S, Dobrev D, Nattel S. Recent advances in the molecular pathophysiology of atrial fibrillation. J Clin Invest 2011;121:2955–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Molina CE, Abu-Taha IH, Wang Q, Roselló-Díez E, Kamler M, Nattel S, Ravens U, Wehrens XHT, Hove-Madsen L, Heijman J, Dobrev D. Profibrotic, electrical, and calcium-handling remodeling of the atria in heart failure patients with and without atrial fibrillation. Front Physiol 2018;9:1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Grammatika PN, Dobrev S, Beneke K, Reinhardt F, Pecha S, Jacquet E, Abu-Taha IH, Schmidt C, Voigt N, Kamler M, Schnabel RB, Baczkó I, Garnier A, Reichenspurner H, Nikolaev VO, Dobrev D, Molina CE. Phosphodiesterase 8 governs cAMP/PKA-dependent reduction of L-type calcium current in human atrial fibrillation: a novel arrhythmogenic mechanism. Eur Heart J 2023;44(27):2483–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Voigt N, Heijman J, Wang Q, Chiang DY, Li N, Karck M, Wehrens XHT, Nattel S, Dobrev D. Cellular and molecular mechanisms of atrial arrhythmogenesis in patients with paroxysmal atrial fibrillation. Circulation 2014;129:145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Gaborit N, Steenman M, Lamirault G, Le Meur N, Le Bouter S, Lande G, Léger J, Charpentier F, Christ T, Dobrev D, Escande D, Nattel S, Demolombe S. Human atrial ion channel and transporter subunit gene-expression remodeling associated with valvular heart disease and atrial fibrillation. Circulation 2005;112:471–481. [DOI] [PubMed] [Google Scholar]
- 110. Klein G, Schröder F, Vogler D, Schaefer A, Haverich A, Schieffer B, Korte T, Drexler H. Increased open probability of single cardiac L-type calcium channels in patients with chronic atrial fibrillation. Role of phosphatase 2A. Cardiovasc Res 2003;59:37–45. [DOI] [PubMed] [Google Scholar]
- 111. Qi XY, Yeh Y-H, Xiao L, Burstein B, Maguy A, Chartier D, Villeneuve LR, Brundel BJJM, Dobrev D, Nattel S. Cellular signaling underlying atrial tachycardia remodeling of L-type calcium current. Circ Res 2008;103:845–854. [DOI] [PubMed] [Google Scholar]
- 112. Alsina KM, Hulsurkar M, Brandenburg S, Kownatzki-Danger D, Lenz C, Urlaub H, Abu-Taha I, Kamler M, Chiang DY, Lahiri SK, Reynolds JO, Quick AP, Scott L, Word TA, Gelves MD, Heck AJR, Li N, Dobrev D, Lehnart SE, Wehrens XHT. Loss of protein phosphatase 1 regulatory subunit PPP1R3A promotes atrial fibrillation. Circulation 2019;140:681–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Wittköpper K, Fabritz L, Neef S, Ort KR, Grefe C, Unsöld B, Kirchhof P, Maier LS, Hasenfuss G, Dobrev D, Eschenhagen T, El-Armouche A. Constitutively active phosphatase inhibitor-1 improves cardiac contractility in young mice but is deleterious after catecholaminergic stress and with aging. J Clin Invest 2010;120:617–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Dobrev D, Friedrich A, Voigt N, Jost N, Wettwer E, Christ T, Knaut M, Ravens U. The g protein–gated potassium current ik, ach is constitutively active in patients with chronic atrial fibrillation. Circulation 2005;112:3697–3706. [DOI] [PubMed] [Google Scholar]
- 115. Dobrev D, Graf E, Wettwer E, Himmel HM, Hála O, Doerfel C, Christ T, Schüler S, Ravens U. Molecular basis of downregulation of G-protein-coupled inward rectifying K+ current (IK. ACh) in chronic human atrial fibrillation. Circulation 2001;104:2551–2557. [DOI] [PubMed] [Google Scholar]
- 116. Voigt N, Trausch A, Knaut M, Matschke K, Varró A, Wagoner DRV, Nattel S, Ravens U, Dobrev D. Left-to-right atrial inward rectifier potassium current gradients in patients with paroxysmal versus chronic atrial fibrillation. Circ Arrhythm Electrophysiol 2010;3:472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Pereira L, Cheng H, Lao DH, Na L, van Oort RJ, Brown JH, Wehrens XHT, Chen J, Bers DM. Epac2 mediates cardiac β1-adrenergic dependent SR Ca2+ leak and arrhythmia. Circulation 2013;127:913–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Pereira L, Métrich M, Fernández-Velasco M, Lucas A, Leroy J, Perrier R, Morel E, Fischmeister R, Richard S, Bénitah J-P, Lezoualc’h F, Gómez AM. The cAMP binding protein Epac modulates Ca2+ sparks by a Ca2+/calmodulin kinase signalling pathway in rat cardiac myocytes. J Physiol 2007;583:685–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Pereira L, Bare DJ, Galice S, Shannon TR, Bers DM. β-Adrenergic induced SR Ca2+ leak is mediated by an epac-NOS pathway. J Mol Cell Cardiol 2017;108:8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Anderson ME, Brown JH, Bers DM. CaMKII in myocardial hypertrophy and heart failure. J Mol Cell Cardiol 2011;51:468–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Erickson JR, Pereira L, Wang L, Han G, Ferguson A, Dao K, Copeland RJ, Despa F, Hart GW, Ripplinger CM, Bers DM. Diabetic hyperglycaemia activates CaMKII and arrhythmias by O-linked glycosylation. Nature 2013;502:372–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Erickson JR, Nichols CB, Uchinoumi H, Stein ML, Bossuyt J, Bers DM. S-nitrosylation induces both autonomous activation and inhibition of calcium/calmodulin-dependent protein kinase II δ. J Biol Chem 2015;290:25646–25656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Neef S, Heijman J, Otte K, Dewenter M, Saadatmand AR, Meyer-Roxlau S, Antos CL, Backs J, Dobrev D, Wagner M, Maier LS, El-Armouche A. Chronic loss of inhibitor-1 diminishes cardiac RyR2 phosphorylation despite exaggerated CaMKII activity. Naunyn Schmiedebergs Arch Pharmacol 2017;390:857–862. [DOI] [PubMed] [Google Scholar]
- 124. Blitzer RD, Connor JH, Brown GP, Wong T, Shenolikar S, Iyengar R, Landau EM. Gating of CaMKII by cAMP-regulated protein phosphatase activity during LTP. Science 1998;280:1940–1942. [DOI] [PubMed] [Google Scholar]
- 125. Takanari H, Fontes MSC, van der Heyden MAG, Vos MA, van Veen TAB. Response to the letter from Warren. Cardiovasc Res 2017;113:1799–1800. [DOI] [PubMed] [Google Scholar]
- 126. Warren M, Zaitsev AV. CaMKII blockade, cardiac conduction, and arrhythmia. Cardiovasc Res 2017;113:1798–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Zaitsev AV, Torres NS, Cawley KM, Sabry AD, Warren JS, Warren M. Conduction in the right and left ventricle is differentially regulated by protein kinases and phosphatases: implications for arrhythmogenesis. Am J Physiol Heart Circ Physiol 2019;316:H1507–H1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Ai X, Pogwizd SM. Connexin 43 downregulation and dephosphorylation in nonischemic heart failure is associated with enhanced colocalized protein phosphatase type 2A. Circ Res 2005;96:54–63. [DOI] [PubMed] [Google Scholar]
- 129. Burstein B, Comtois P, Michael G, Nishida K, Villeneuve L, Yeh Y-H, Nattel S. Changes in connexin expression and the atrial fibrillation substrate in congestive heart failure. Circ Res 2009;105:1213–1222. [DOI] [PubMed] [Google Scholar]
- 130. Darrow BJ, Fast VG, Kléber AG, Beyer EC, Saffitz JE. Functional and structural assessment of intercellular communication. Circ Res 1996;79:174–183. [DOI] [PubMed] [Google Scholar]
- 131. Moreno AP, Sáez JC, Fishman GI, Spray DC. Human connexin43 gap junction channels. Regulation of unitary conductances by phosphorylation. Circ Res 1994;74:1050–1057. [DOI] [PubMed] [Google Scholar]
- 132. Heijman J, Ghezelbash S, Wehrens XHT, Dobrev D. Serine/threonine phosphatases in atrial fibrillation. J Mol Cell Cardiol 2017;103:110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Heijman J, Dewenter M, El-Armouche A, Dobrev D. Function and regulation of serine/threonine phosphatases in the healthy and diseased heart. J Mol Cell Cardiol 2013;64:90–98. [DOI] [PubMed] [Google Scholar]
- 134. Zhang X, Ni H, Morotti S, Smith CER, Sato D, Louch WE, Edwards AG, Grandi E. Mechanisms of spontaneous Ca2+ release-mediated arrhythmia in a novel 3D human atrial myocyte model: I. Transverse-axial tubule variation. J Physiol 2023;601(13):2655–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Heijman J, Dobrev D. Determinants and therapeutic potential of calcium handling abnormalities in atrial fibrillation: what can we learn from computer models? J Physiol 2023;601(13):2545–2546. [DOI] [PubMed] [Google Scholar]
- 136. Kreusser MM, Lehmann LH, Keranov S, Hoting M-O, Oehl U, Kohlhaas M, Reil J-C, Neumann K, Schneider MD, Hill JA, Dobrev D, Maack C, Maier LS, Gröne H-J, Katus HA, Olson EN, Backs J. Cardiac CaM kinase II genes δ and γ contribute to adverse remodeling but redundantly inhibit calcineurin-induced myocardial hypertrophy. Circulation 2014;130:1262–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Parks RJ, Bogachev O, Mackasey M, Ray G, Rose RA, Howlett SE. The impact of ovariectomy on cardiac excitation-contraction coupling is mediated through cAMP/PKA-dependent mechanisms. J Mol Cell Cardiol 2017;111:51–60. [DOI] [PubMed] [Google Scholar]
- 138. Parks RJ, Ray G, Bienvenu LA, Rose RA, Howlett SE. Sex differences in SR Ca2+ release in murine ventricular myocytes are regulated by the cAMP/PKA pathway. J Mol Cell Cardiol 2014;75:162–173. [DOI] [PubMed] [Google Scholar]
- 139. Li Z, Mirams GR, Yoshinaga T, Ridder BJ, Han X, Chen JE, Stockbridge NL, Wisialowski TA, Damiano B, Severi S, Morissette P, Kowey PR, Holbrook M, Smith G, Rasmusson RL, Liu M, Song Z, Qu Z, Leishman DJ, Steidl-Nichols J, Rodriguez B, Bueno-Orovio A, Zhou X, Passini E, Edwards AG, Morotti S, Ni H, Grandi E, Clancy CE, Vandenberg J, Hill A, Nakamura M, Singer T, Polonchuk L, Greiter-Wilke A, Wang K, Nave S, Fullerton A, Sobie EA, Paci M, Tshinanu FM, Strauss DG. General principles for the validation of proarrhythmia risk prediction models: an extension of the CiPA in silico strategy. Clin Pharmacol Ther 2020;107:102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Passini E, Britton OJ, Lu HR, Rohrbacher J, Hermans AN, Gallacher DJ, Greig RJH, Bueno-Orovio A, Rodriguez B. Human in silico drug trials demonstrate higher accuracy than animal models in predicting clinical pro-arrhythmic cardiotoxicity. Front Physiol 2017;8:668. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material. Source codes, analysis scripts, and related parameter data are available for download at elegrandi.wixsite.com/grandilab/downloads and github.com/drgrandilab.