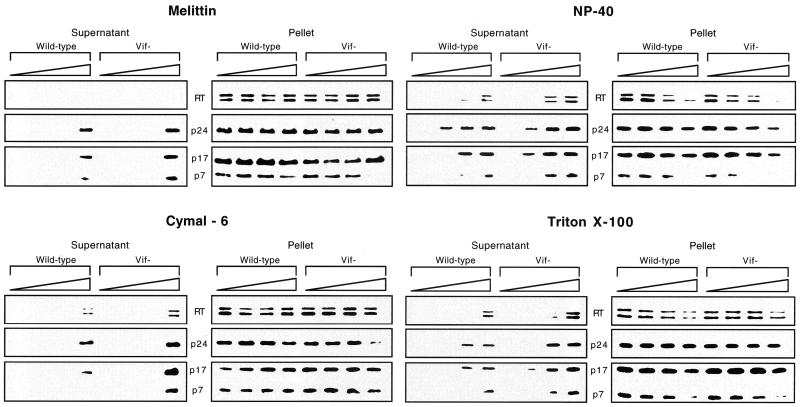
FIG. 2.
Release of core proteins into the soluble fraction after detergent treatment of wild-type and vif mutant virions. Wild-type and vif mutant virions (200,000 cpm of exogenous RT units per sample) produced in CEM cells were permeabilized with melittin (0, 5, 10, and 20 μg/ml), NP-40 (0, 0.01, 0.02, and 0.04%), Cymal-6 (0, 0.01, 0.02, and 0.04%), or Triton X-100 (0, 0.01, 0.02, and 0.04%) prior to addition of buffer and incubation for 1 h at 37°C. The samples were centrifuged, and the supernatant and pellet fractions were separated by SDS-PAGE and analyzed by Western blotting. The blots were used for multiple hybridizations and were initially hybridized with a rabbit anti-p24 antibody. The blots were then stripped and reprobed sequentially with mouse anti-RT followed by a mixture of mouse anti-p17 and anti-p7 monoclonal antibodies. Results are representative of two or three independent experiments using different preparations of virions.