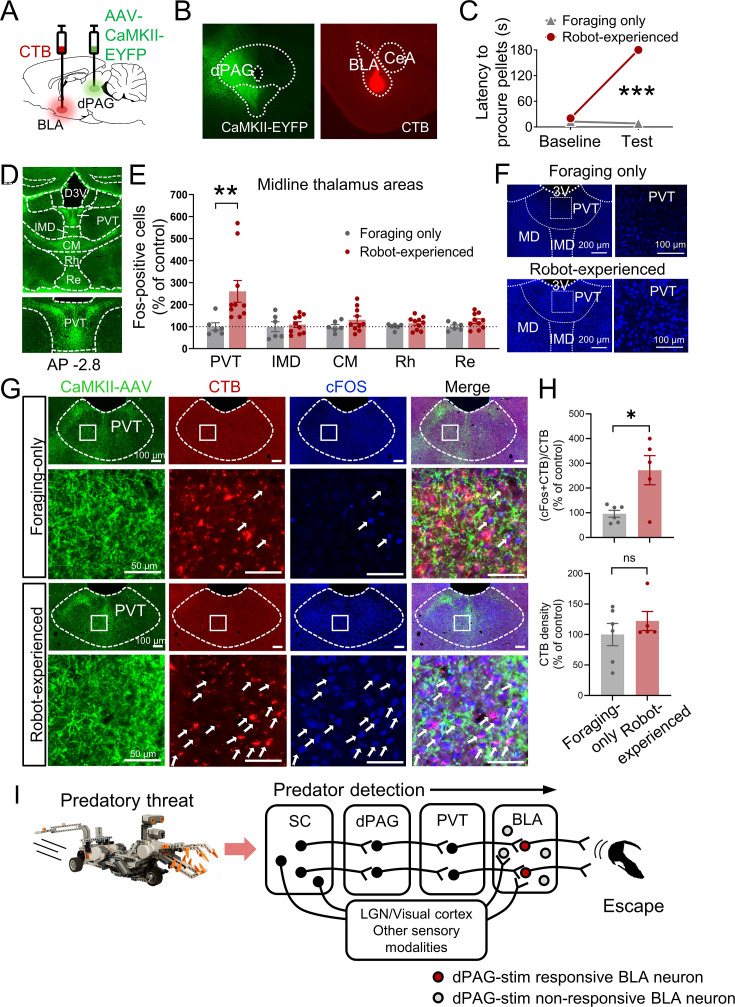
Figure 4. Paraventricular nucleus of the thalamus’ (PVT) hypothesized role in dorsal periaqueductal gray (dPAG) to amygdala signaling and antipredatory behavior model.
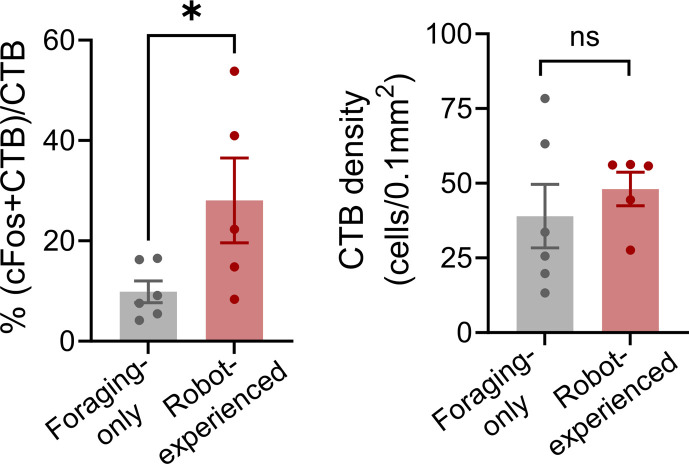
(A) Cholera toxin subunit B (CTB) retrograde tracer and AAV-CaMKII-EYFP used to trace dPAG signals to the basolateral amygdala (BLA). (B) Representative images of AAV and CTB expressions in dPAG and BLA, respectively. (C) Robot encounters hindered pellet procurement compared to foraging-only rats (Base, U = 26, p=0.6926; Test, U = 0, p=0.0001; Mann–Whitney U test). ***p<0.001 compared to the foraging-only group. (D) Terminal expressions of AAV injected into the dPAG cell bodies were predominantly observed in the midline nuclei of the thalamus. (E) PVT showed higher c-Fos-positive cells in robot-experienced rats (n = 10) compared to foraging-only control rats (n = 6) (U = 4.0, p=0.0027; Mann–Whitney U test), while other midline thalamic areas showed no differences (t(14)s < 1.611, ps>0.129; t-test). **p<0.01 compared to the foraging-only group. Values were normalized to the mean of the corresponding control group. (F) Representative photomicrographs of PVT c-Fos staining from foraging-only (upper) and robot-experienced (bottom) rats. (G) Representative microphotographs of AAV, CTB, c-Fos, and triple staining in PVT comparing foraging-only and robot-experienced animals. (H) Robot exposure increased the percentage of CTB-labeled PVT neurons expressing c-Fos (t(9) = 3.171, p=0.0113), while CTB density levels were comparable between the two groups (robot-experienced, n = 5; foraging-only, n = 6; t(9) = 0.9039, p=0.3896; t-test). Values were normalized to the mean of the corresponding control group. (I) Proposed model: predator surge detection via visual pathways, e.g., superior colliculus (Furigo et al., 2010; Rhoades et al., 1989), leads to dPAG activation, signaling through PVT to excite BLA. The BLA then projects to regions controlling escape responses, such as dorsal/posterior striatum (Li et al., 2021; Menegas et al., 2018) and ventromedial hypothalamus (Silva et al., 2013; Kunwar et al., 2015; Wang et al., 2015).