Abstract
Context
Carriers of germline pathogenic variants (PVs) in succinate dehydrogenase type B (SDHB) are at increased risk of developing pheochromocytomas and paragangliomas (PPGLs). Understanding their outcomes can guide recommendations for risk assessment and early detection.
Objective
We performed a systematic review and meta-analysis of the following outcomes in SDHB PV carriers: age-specific risk of developing tumors, metastatic progression, second primary tumor development, and mortality.
Methods
PubMed, MEDLINE, and EMBASE were searched. Sixteen studies met the inclusion criteria and were sorted into 4 outcome categories: age-specific penetrance, metastatic disease, risk of second tumor, and mortality. We assessed heterogeneity and performed a meta-analysis across studies using a random-effects model with the DerSimonian and Laird method.
Results
Penetrance of PPGLs for nonproband/nonindex SDHB PV carriers by age 20 was 4% (95% CI, 3%-6%), 11% (95% CI, 8%-15%) by age 40, 24% (95% CI, 19%-31%) by age 60%, and 35% (95% CI, 25%-47%) by age 80. The overall risk of metastatic disease for nonproband/nonindex carriers with PPGLs was 9% (95%, CI 5%-16%) per lifetime. In all affected cases (combining both proband/index and nonproband/nonindex carriers with tumors), the risk of a second tumor was 24% (95% CI, 18%-31%) and all-cause 5-year mortality was 18% (95% CI, 6%-40%).
Conclusion
Penetrance for PPGLs in SDHB PV carriers increases linearly with age. Affected carriers are at risk of developing and dying of metastatic disease, or of developing second tumors. Lifelong surveillance is appropriate.
Keywords: SDHB, succinate dehydrogenase, pheochromocytoma, paraganglioma
Carriers of germline pathogenic variants (PVs) in succinate dehydrogenase type B (SDHB) are at increased risk of developing pheochromocytomas and paragangliomas (PPGLs) (1). Pheochromocytomas arise from chromaffin cells in the adrenal medulla, and paragangliomas arise from chromaffin cells in sympathetic, or chief cells in parasympathetic, ganglia (2). Overall, around 20% of all PPGL cases are attributed to germline PVs in genes encoding SDH subunits (SDHx), with the most frequent being SDHB (3). SDHB PV carriers are typically identified after a proband/index patient presents with PPGL and undergoes genetic testing, followed by family risk notification and predictive testing.
SDHB PV carriers have a significantly increased risk of metastatic progression and mortality. A recent analysis of 448 proband cases from a registry data set noted that metastatic disease affected 27% of SDHB PV carriers (4). In another paper, proband patients were 4 times more likely to develop metastatic disease compared with nonproband cases (5). A recent analysis of PPGLs from the Cancer Genome Atlas found that patients with PPGLs associated with a germline SDHB PV had significantly higher 15-year mortality (HR = 4.7), compared to non-SDHB cases (6).
Understanding the outcomes facing this group is clinically important, as it can help to inform patients, their families, and health-care providers regarding risk assessment, early detection, and intervention strategies. Previous systematic reviews and meta-analyses (7-9) have not focused on outcomes facing nonproband/nonindex SDHB PV carriers. The risk of metastatic progression for nonindex SDHB PV carriers was last assessed in a systematic review and meta-analysis more than a decade ago (10), and in light of subsequent studies deserves an update. The aim of this study was to assess the following outcomes in SDHB PV carriers by meta-analysis of published studies in the field: age-specific risk of developing tumors, risk of metastatic progression, risk of developing a second primary tumor, and risk of death. An understanding of these risks is crucial for optimizing patient management and improving clinical outcomes, because these 4 risks are key factors in driving the high burden of disease facing SDHB PV carriers and health-care utilization. Tumor development is associated with symptoms of catecholamine excess and/or mass-effect symptoms to surrounding structures (11); each subsequent tumor increases the likelihood of morbidity and mortality; metastatic progression is associated with morbidity from tumor symptoms (11) and is a risk factor for mortality (12, 13).
Materials and Methods
Eligibility Criteria
We searched for original retrospective and prospective observational studies reporting on outcomes for SDHB PV carriers whose carrier status was confirmed on genetic testing. Studies were included if they reported on any of the following outcome measures: (1) age-specific penetrance (age 20, 40, 60, and/or 80 years), (2) number or proportion of SDHB PV carriers who developed metastatic disease, (3) number or proportion of SDHB PV carriers who developed second tumor(s), and (4) mortality of SDHB PV carriers with metastatic disease. Penetrance was defined as the proportion of SDHB PV carriers who developed PPGL. Metastasis was defined by World Health Organization classification as the presence of chromaffin tissue in nonchromaffin organs such as lymph nodes, liver, lungs, and bone (2). In the event a study reported metastasis on imaging without a biopsy diagnosis, we categorized this as metastasis and not as a second tumor. We classified second tumor only when a study used the phrases “second tumor” or “multifocal” or provided individual patient data on the particular second tumor diagnoses (the type of head or neck paraganglioma [HNPGL] or thoracoabdominal paragangliomas and pheochromocytomas [TAPPGLs]). Mortality was defined as the number of patients who died of the disease. For mortality estimates we included studies that reported 5-year mortality of SDHB PV carriers with metastatic disease to minimize bias from studies with unclear or short duration of follow-up. We did not exclude studies that reported on SDHB PV carriers who presented with symptomatic disease. We also did not exclude studies with probands, defined as the first individual in a family to be diagnosed with an SDHB PV after presenting with a tumor, nor did we exclude studies with index cases, defined as the first identified case of SDHB PV. However, if data on nonproband/nonindex SDHB PV carriers were available, we assessed these results preferentially to data on probands/index carriers. We only included results for nonproband/nonindex cases for the outcomes of age-specific penetrance and risk of metastatic progression. However, for risk of second tumor, we found only one study that reported nonproband/nonindex carrier data, so we could not assess nonproband/nonindex carrier data preferentially. Similarly for mortality risk, we found only one study that reported nonproband/nonindex carrier 5-year mortality data, so we could not assess nonproband/nonindex carrier data preferentially.
As a systematic review and meta-analysis has been performed looking at the risk of metastatic disease for the combined group of proband/index and nonproband/nonindex SDHB PV carriers (9), we performed a meta-analysis of the risk of metastatic progression for nonproband/nonindex SDHB PV carriers with disease only. Studies with fewer than 10 SDHB PV carriers were excluded. Where studies reported on the same cohort of patients, we assessed the study with the larger number of SDHB PV carriers addressing the outcome of interest.
Search Strategy
In March 2022 the databases PubMed, Ovid MEDLINE, and Ovid EMBASE were searched by D.F.D. Studies were limited to those in humans but there was no limit on language. Additional records were identified through primary article references. The PubMed search was as follows: ((paraganglioma or pheochromocytoma) and (succinate or SDHB or SDH)) limited to humans. The Ovid MEDLINE search was as follows: (succinates/or succinate.mp. or SDHB.mp.) and (exp paraganglioma/or paraganglioma.mp.) limited to humans. The Ovid EMBASE search was as follows: (exp succinate dehydrogenase/or succinate.mp. or sdhb.mp. or sdh.mp) and (exp paraganglioma/or exp pheochromocytoma/or paraganglioma.mp or pheochromocytoma) limited to humans.
Data Selection
Studies from the search were entered into the reference management software (Endnote X9). Duplicates were removed and articles were screened for eligibility based on title and abstract by D.F.D. Basic science reports, case reports, review articles, editorials, conference abstracts with insufficient data, nonoriginal research, and unrelated articles (those that did not address the research questions or meet the inclusion criteria) were removed. Once relevant full-text articles were obtained, a full-text review of screened articles was conducted by authors D.F.D., D.E.B., V.H.M.T., and R.C.B. with disagreements determined by group consensus with R.D.A.L. We performed an analysis of the penetrance data from our cohort (5) (Appendix 1 (14)) and included the data in the pooled penetrance assessment.
Articles were separated into 4 categories according to penetrance for nonproband/nonindex SDHB PV carriers, risk of metastatic disease for SDHB PV carriers with disease, risk of a second tumor for SDHB PV carriers with disease, and 5-year mortality for SDHB PV carriers with metastatic disease.
Data Extraction
The following data were extracted from eligible articles: first author, year of publication, observational study design, country, data collection, duration of follow-up, number of participants who were nonproband/nonindex SDHB PV carriers, number of nonproband/nonindex SDHB PV carriers who developed disease by age 20, 40, 60, and/or 80 years based on reported age-specific penetrance, total number of SDHB PV carriers who developed disease, number of SDHB PV carriers who developed metastatic disease, number of SDHB PV carriers who developed a second tumor, and number of SDHB PV carriers with metastatic disease who died within 5 years. Studies were classified as either cohort studies (with follow-up of a cohort of SDHB PV carriers to observe who developed outcomes of interest (15)) or cross-sectional; as retrospective or prospective; and as single-center or multicenter.
Risk of Bias Assessment
The quality of the observational studies was assessed independently by authors D.F.D., D.E.B., and R.C.B. using a modified Newcastle-Ottawa tool described by Hamidi et al (8) and further adapted to this study. The following were described: (1) how the sample represented the population of interest, (2) how genetic information was assessed, (3) how the outcome measures were assessed, (4) sufficient duration of follow-up and, (5) adequacy of follow-up. A detailed description of the tool is listed in the supplementary material (14). The tool used by Hamidi et al (8) was adapted by replacing their question “how the data on metastatic PPGL was collected” with “how genetic information was assessed” because we assessed SDHB PV carriers only and we assessed 4 outcomes rather than the 1 outcome of metastatic disease risk.
Statistical Analyses
Penetrance in our cohort of SDHB PV carriers (5) was performed using the Kaplan-Meier method. The outcome of the meta-analysis was the pooled penetrance of PPGL in SDHB PV carriers. The proportion of patients with PPGLs by age 20, 40, 60, and 80 years was calculated as the number of patients with PPGLs at those ages divided by the total number of SDHB PV carriers. Probands/Index cases were excluded to reduce ascertainment bias (16). For all 4 outcomes of interest, a meta-analysis of proportions was performed by using a random-effects generalized linear mixed model due to expected differences in the populations from which data was pooled. The random-effects model was determined using the DerSimonian and Laird method (17), with the estimate of heterogeneity taken from the inverse variance method. CIs were obtained using the Jackson method. Prediction intervals were calculated to denote the penetrance that may be observed in future studies. Publication bias assessment was attempted but ultimately could not be assessed with Egger's regression test, funnel plot asymmetry or the meta-regression “weightr” package in R, because there were too few studies and insufficient data to run these tests, defined as fewer than 10 studies for each pooled assessment (18). Analyses were performed with R version 4.2.2.
Results
Study Selection
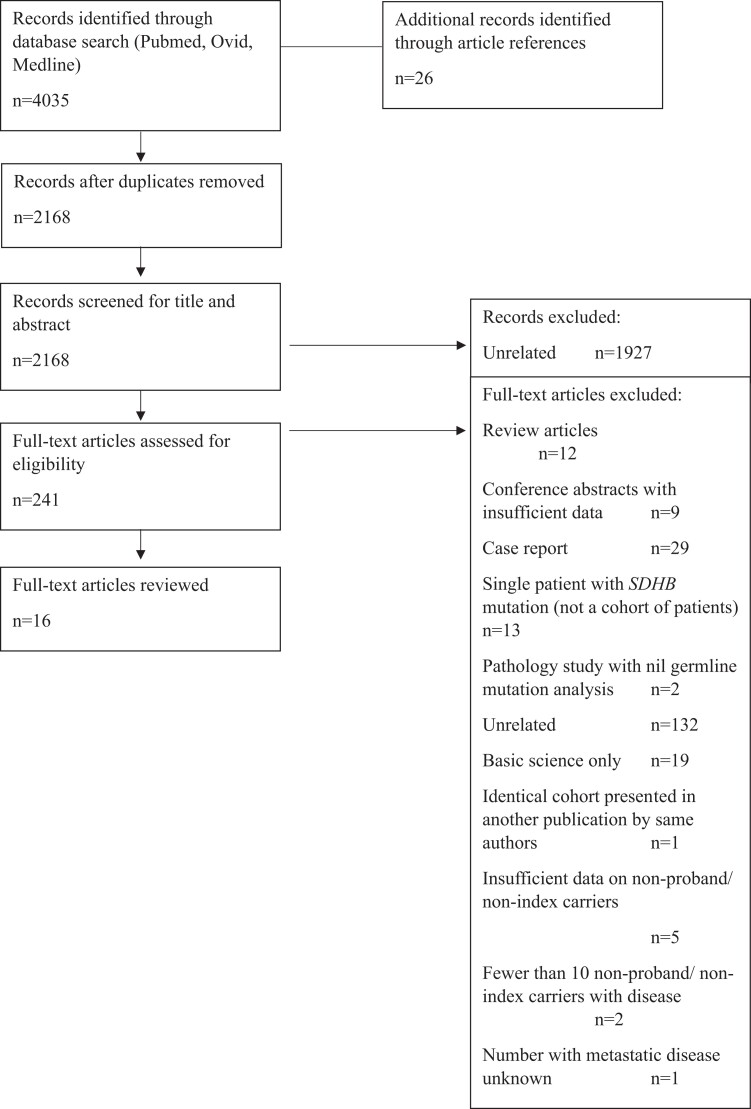
The search of publications produced 4035 references. Duplicates were removed and abstracts reviewed, leaving 241 publications for assessment. The manuscripts were reviewed and a further 224 publications were removed: A total of 12 publications were eliminated due to being review articles, 9 publications were conference abstracts, 29 studies were case reports, 13 studies were case reports on SDHB PV carriers, 2 were pathology studies, 132 publications were unrelated, and 19 were basic science publications. One study was an identical cohort presented in another publication by the same authors. Two studies reported risk of metastatic disease but were not included in the assessment because there were fewer than 10 nonproband/nonindex SDHB PV carriers with disease. Five studies that reported risk of metastatic disease were excluded because there were insufficient data on nonproband/nonindex SDHB PV carriers. One study reported 5-year mortality but did not report the number of SDHB PV carriers with metastatic disease. Altogether, 16 studies met the inclusion criteria (5,12, 13, 16, 19-30). The study inclusion flow diagram is shown in Fig. 1.
Figure 1.
Study flow diagram.
Study Characteristics
Of the 16 studies, 10 were cohort studies (5, 12, 13, 19-21, 23, 26, 28, 30), and 6 were cross-sectional studies (16, 22, 24, 25, 27, 29) (Table 1). The follow-up duration of cohort studies was variable and median follow-up ranged between 1 year and 5.9 years. Twelve studies were retrospective, 3 were prospective, and 1 study was retrospective with ongoing prospective follow-up. Nine were single-center and 7 were multicenter. Seven studies were suitable for the penetrance assessment, 5 for assessing risk of metastatic disease assessment, 5 for assessing risk of a second tumor, and 6 for the 5-year mortality of SDHB PV carriers with metastatic disease assessment. The number of SDHB PV carriers in each study ranged from 11 to 317.
Table 1.
Characteristics of studies
| Author | Year | Country | Study design | Population studied | Data collection | Follow-up | Outcome category of this meta-analysis | No. of nonproband/nonindex SDHB PV carriers | Penetrance of SDHB PV carriers with PPGL and/or HNPGL (probands/index cases excluded where known) | Total No. of SDHB PV carriers with PPGL and/or HNPGL | No. of SDHB PV carriers with multifocal disease or second tumor (excluding metastatic disease) | Total No. of SDHB PV carriers with metastatic disease | 5-y survival of SDHB PV carriers with metastatic disease |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amar et al | 2007 | France | Retrospective multicenter cohort study | Patients with metastatic PPGL | NA-2005 | For SDHB carrier, median follow-up was 28 mo | Mortality | NA | NA | 23 | NA | 23 | 36% |
| Andrews et al | 2018 | UK | Retrospective multicenter cross-sectional study | SDHB/SDHC/SDHD carriers | NA | NA | Penetrance, metastatic disease | 371 | Age 60: 83; age 80: 145a | NA | NA | 9 | NA |
| Bausch et al | 2014 | Germany, Italy, Poland, France, UK, Hungary, Ukraine, Latvia, Argentina, USA | Prospective multicenter cohort study | Patients with symptomatic paraganglial tumors | NA-2013 | 10 y (range, 1-42 y) for SDHB carriers | Second tumor | NA | NA | 25 | 6 | 2 | NA |
| Daniel et al | 2016 | UK | Retrospective single-center cohort study |
SDHx
PV carriers |
2005-2015 | 6.4 y (range, 3.1-10.0 y) | Second tumor | 27 | NA | 11 | 2 | 1 | NA |
| Davidoff et al | 2022 (this study for penetrance assessment) | Australia | Retrospective and ongoing prospective multicenter cohort study |
SDHB
PV carriers |
1994-2021 | Median 5.9 y (range, 1 mo-23.9 y) | Penetrance, metastatic disease, second tumor, Mortality | 148 | Age 20: 7; age 40: 17; age 60: 25; age 80: 29b | 62 | 18 | 3 | 69% |
| Eijkelenkamp et al | 2017 | Netherlands | Retrospective single-center cohort study | SDHB PV carriers | 2008-2015 | Median 3.3 y (IQR 2.2-4.5) | Penetrance | 70 | Age 40: 1; age 60: 8c | NA | NA | 8 | NA |
| Jafri et al | 2013 | UK | Prospective multicenter cross-sectional study | SDHB probands who presented with PPGL and/or HNPGL and nonproband carriers | 2001-2011 | NA | Penetrance | 187 | Age 20: 9; age 40: 30; age 60: 75d | NA | NA | NA | NA |
| Jochmanova et al | 2017 | USA | Retrospective single-center cohort study | Family members of SDHB index cases who presented with PPGL | 2004-2016 | Median 1 y (range 0-14 y) | Penetrance, metastatic disease | 241 | Age 20: 8; age 40: 30; age 60: 64; age 80: 118e | 143 | NA | 7 | NA |
| Jochmanova et al | 2020 | USA | Retrospective single-center cross-sectional study | SDHB PV carriers with PPGL | 2000-2019 | NA | Mortality | NA | NA | 64 | NA | 45 | 100% |
| King et al | 2011 | USA | Retrospective single-center cross-sectional study | Patients with metastatic PPGL | 2000-2010 | NA | Mortality | NA | NA | 23 | NA | 23 | 96% |
| Niemeijer et al | 2017 | Netherlands | Retrospective multicenter cohort study | SDHB carriers | Prior to 2014 | Median 2.6 y (range, 0-36 y) | Penetrance, metastatic disease | 129 | Age 20: 3; age 40: 13; age 60: 32; age 80: 43f | 83 | NA | 15 | NA |
| Schovanek et al | 2014 | USA | Retrospective single-center cross-sectional study | Patients with SDHB-related PPGL | NA | NA | Mortality | NA | NA | NA | NA | 77 | 76% |
| Srirangalingam et al | 2008 | UK | Retrospective multicenter cohort study | SDHB PV carriers | NA | Mean follow-up of 5.8 y (SD 7.4, range 0-31 y) | Second tumor | NA | NA | 16 | 3 | NA | NA |
| Tufton et al | 2017 | UK | Prospective single-center cohort study | SDHB PV carriers | 1975-2015 | Mean follow-up 5.7 y (range, 0-14 y) | Second tumor, metastatic disease | 65 | NA | 40 | 8 | 8 | NA |
| Turkova et al | 2016 | USA | Retrospective single-center cross-sectional study | Patients with metastatic PPGL | 2000-2015 | NA | Mortality | NA | NA | 73 | NA | 73 | 92% |
| White et al | 2022 | UK | Retrospective single-center cohort study | SDHx carriers who presented with PPGL and first-degree relatives with SDHx PV | 2000-2020 | Median 3 y | Penetrance | 56 | Age 20: 1; age 40: 3; age 60: 16g | NA | NA | NA | NA |
Abbreviations: HNPGL, head and neck paraganglioma; IQR, interquartile range; NA, not applicable; na, not available; PPGL, pheochromocytoma and paraganglioma; PV, pathogenic variant; SDHB, succinate dehydrogenase type B; SDHx, succinate dehydrogenase; UK, United Kingdom; USA, United States of America.
a-g Penetrance figures are based on the assumption that all nonproband/nonindex SDHB PV carriers were included in the age-specific penetrance proportions reported for nonproband/nonindex carriers in each study.
a Andrews et al (2013): age 60: 22.5%; age 80: 39%.
b Davidoff et al (this study): age 20: 5%; age 40: 11%; age 60: 17%; age 80: 20%.
c Eijkelenkamp et al (2017): age 40: 2%; age 60: 12%.
d Jafri et al (2013): age 20: 5%; age 40: 16%; age 60: 40%.
e Jochmanova et al (2017): age 20: 3.3%; age 40: 12.4%; age 60: 26.4%; age 80: 48.8%.
f Niemeijer et al (2017): age 20: 2%; age 40: 10%; age 60: 25%; age 80: 33%.
g White et al (2022): age 20: 2.5%; age 40: 5%; age 60: 28.7%.
Risk of Bias Assessment
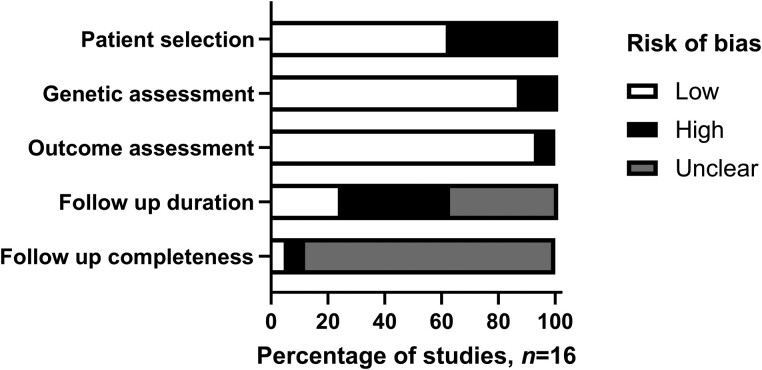
Risk of bias assessments were performed for each study, and results are summarized in Fig. 2. Patient selection assessment was assessed as having a low risk of bias for 10 studies and a high risk of bias for 6 studies. Genetic diagnosis (14/16) and clinical outcomes (15/16) were assessed by our authors as having a low risk of bias. Follow-up duration (12/16 high or unclear risk) and follow-up completeness (15/16 high or unclear risk) were assessed as having high risk of bias.
Figure 2.
Risk of bias assessment.
Penetrance for Nonproband/Nonindex SDHB Pathogenic Variant Carriers: Meta-Analysis
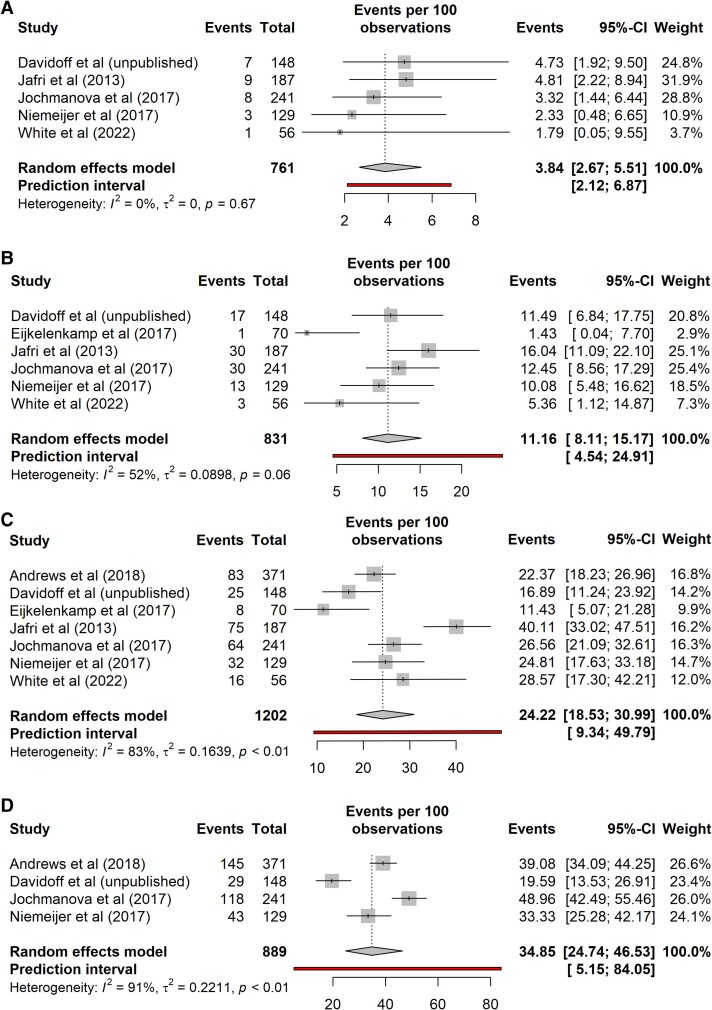
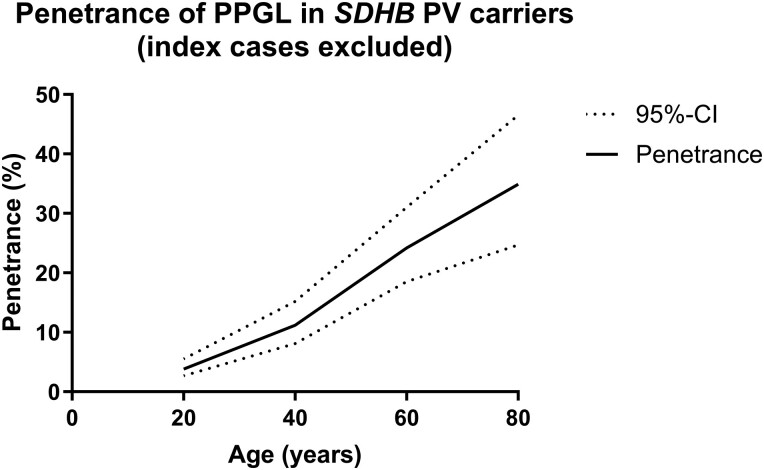
Results for age-specific penetrance for nonproband/nonindex SDHB PV carriers are shown in Fig. 3. The pooled penetrance of PPGL by age 20 was 4% (95% CI, 3%-6%; prediction interval, 2%-7%; n = 761, 5 studies) with I2 of 0%. By age 40 the pooled penetrance was 11% (95% CI, 8%-15%; prediction interval, 5%-25%; n = 831, 6 studies). There was moderate variability between studies with I2 of 52%. By age 60 pooled penetrance was 24% (95% CI, 19%-31%; prediction interval, 9%-50%; n = 1202, 7 studies). There was high variability between studies with I2 of 83%. By age 80 pooled penetrance was 35% (95% CI, 25%-47%; prediction interval, 5%-84%; n = 889, 4 studies). There was high variability between studies with I2 of 91%. The overall pooled penetrance is summarized in Fig. 4. Excluding the penetrance data from our cohort (5) produced similar results for pooled penetrance and heterogeneity at age 20, 40, and 60 years but gave a higher penetrance for age 80 years (Appendices 1 and 2) (14).
Figure 3.
Penetrance of pheochromocytomas and paragangliomas for nonproband/nonindex SDHB pathogenic variant carriers. A, Age 20 years. B, Age 40 years. C, Age 60 years. D, Age 80 years.
Figure 4.
Overall pooled penetrance of pheochromocytomas and paragangliomas (PPGL) for SDHB pathogenic variant (PV) carriers, excluding proband/index cases.
Risk of Metastatic Disease for Nonproband/Nonindex SDHB Pathogenic Variant Carriers With Disease: Meta-Analysis
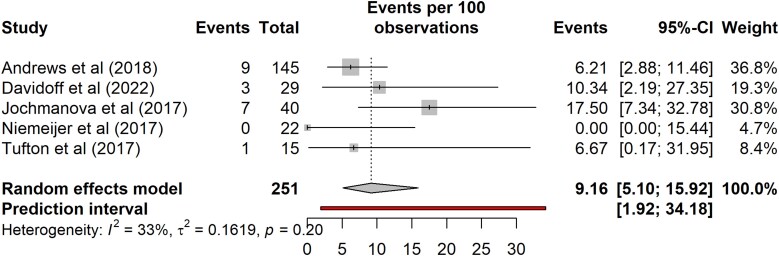
Results for the risk of metastatic disease for SDHB PV carriers with disease, excluding proband/index cases, are shown in Fig. 5. The pooled risk of metastatic disease for nonproband/nonindex SDHB PV carriers with tumors was 9% (95% CI, 5%-16%; prediction interval 2%-34%; n = 251, 5 studies). There was mild variability between studies with I2 of 33%. The subgroup analysis of risk of metastatic disease in nonproband/nonindex carriers with HNPGLs and TAPPGLs are provided in Supplementary Fig. S1 (14).
Figure 5.
Risk of metastatic disease for SDHB pathogenic variant carriers with disease, excluding proband/index cases.
Risk of a Second Tumor for SDHB Pathogenic Variant Carriers With Disease: Meta-Analysis
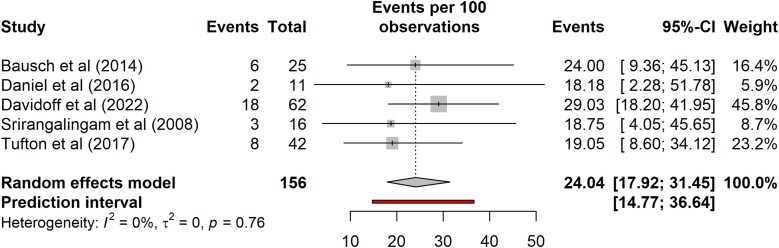
The risk of developing a second primary tumor could not be determined for nonindex carriers alone due to insufficient data in the literature. Considering outcomes for probands and nonprobands/nonindex cases with disease combined, the risk of a second primary tumor is shown in Fig. 6. The pooled risk of a second tumor was 24% (95% CI, 18%-31%; prediction interval 15%-37%; n = 156, 5 studies) with I2 of 0%.
Figure 6.
Risk of a second tumor for SDHB pathogenic variant carriers with disease.
Five-Year Mortality for SDHB Pathogenic Variant Carriers With Metastatic Disease: Meta-Analysis
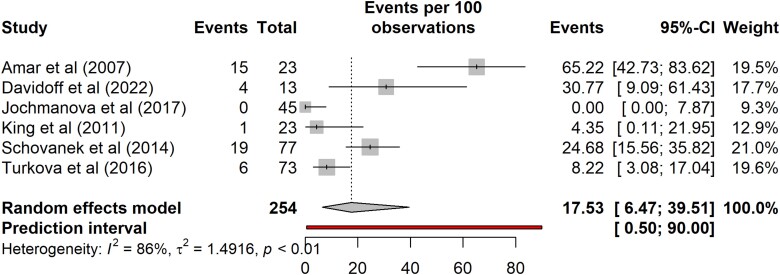
Five-year mortality could not be determined for nonindex carriers alone due to insufficient data in the literature. Combining outcomes for probands and nonprobands/nonindex cases with metastatic disease is shown in Fig. 7. Five-year mortality of SDHB PV carriers with metastatic disease was 18% (95% CI, 6%-40%; prediction interval 1%-90%; n = 254, 6 studies). There was high variability between studies with I2 of 86%.
Figure 7.
Five-year mortality of SDHB pathogenic variant carriers with metastatic disease.
Discussion
In this systematic review and meta-analysis, we aimed to assess 4 clinically relevant outcomes facing SDHB PV carriers, namely, penetrance of disease, risk of metastatic progression, risk of developing a second tumor, and mortality following a diagnosis of metastatic disease. These outcomes were selected due to their relevance to patients, families, and health-care providers conducting surveillance and treatment.
To our knowledge, this is the first systematic review and meta-analysis to examine penetrance for SDHB PV carriers excluding proband/index cases, to assess the risk of developing second tumor, and to report on 5-year mortality of SDHB PV carriers with metastatic disease altogether.
We found penetrance of nonproband/nonindex SDHB PV carriers rose from 4% at age 20 to 24% by age 60 and to 35% by age 80. For nonproband/nonindex SDHB PV carriers who had developed PPGL, overall risk of metastatic progression was 9% per lifetime. Carriers with a history of tumors (proband/index carriers or nonproband/nonindex carriers) had a 24% risk of a second tumor. Finally, all cause 5-year mortality was 18% for both proband/index and nonproband/nonindex carriers with metastatic disease.
Our meta-analysis updates the risk of metastatic progression for SDHB PV carriers excluding proband/index cases. The most recent systematic review and meta-analysis of metastatic disease risk in SDHx PV carriers by Lee et al (9) differed from our assessment, as they included symptomatic and proband/index cases whereas we excluded proband/index cases in the analysis of risk of metastatic progression for SDHB PV carriers who had developed PPGL. Lee et al (9) found a higher risk of 23% of metastatic progression compared to our estimate of 9% in nonproband/nonindex carriers with tumors. We speculate finding a higher risk of metastatic progression when including proband/index cases is likely due to delayed diagnosis and therefore a longer period of “tumor incubation.” In addition, symptomatic patients may have larger tumors, and tumor size is a risk factor for metastatic progression (5, 24, 31).
More than a decade ago, van Hulsteijn et al (10) reported on the risk of malignant (metastatic) paraganglioma in SDHB PV carriers and found the pooled risk in nonindex carriers to be 13%, broadly in keeping with our risk estimate of 9%. In their systematic review and meta-analysis, the heterogeneity of studies was not stated and the investigators cautioned the generalizability of applying the findings, citing the paucity of cohort studies and high risk of bias (10). Our meta-analysis was strengthened by finding only mild variability between studies reporting metastatic progression, with an I2 of 33%; and 4 of the 5 studies in our assessment were cohort studies, compared to 2 of 12 studies in that by van Hulsteijn et al (10).
Hamidi et al (8) performed a systematic review and meta-analysis of overall mortality following a diagnosis of metastatic disease with subgroup analysis of 2 studies reporting on SDHB PV carriers. In our updated analysis we assessed 5-year mortality in 3 times more participants than this previous study (8). We found a lower pooled mortality of 18%, compared to their mortality estimate of 35% to 55% (8). Changes in surveillance practices and treatment options for patients with advanced disease might account for the decrease in mortality estimates.
There were several limitations of the evidence in the pooled analysis. The I2 result of 0% for the studies on pooled penetrance at age 20 years and risk of developing a second tumor suggests there was a potential sampling error (32). While some studies reported their surveillance protocols (5, 13, 20, 21, 24, 26, 28, 30), others did not and differences in follow-up across centers may have modified outcomes reported for SDHB PV carriers. Some of the variants in earlier studies have subsequently been reclassified as variants of uncertain significance (VUS) or likely benign. Of 344 SDHB PV carriers studied by Jochmanova et al (23), 2 index cases in total might have been VUS and 1 index case and 1 nonindex carrier without disease might have been likely benign variants. Of 16 SDHB PV carriers with disease studied by Srirangalingam et al VUS, 1 might have been (28). There was high heterogeneity in the pooled penetrance at age 80 years. There was also high heterogeneity in the 5-year mortality rate of SDHB PV carriers with metastatic disease, and given many of the studies did not describe treatment protocols for carriers with metastatic disease (5, 23, 25, 27, 29), potential treatment differences may have been a factor in variable mortality outcomes. The quality of individual studies included in the systematic review and meta-analysis varied, as shown in the risk of bias assessment. For example, in some studies follow-up duration or completeness was unclear at an individual patient level. Therefore in the pooled analysis of age-specific penetrance, we assumed all nonproband/nonindex SDHB PV carriers were included in the age-specific penetrance proportions reported in each study. Similarly in the pooled analysis of 5-year mortality, we assumed all patients with metastatic disease were included in the 5-year mortality assessment for each study. While these assumptions would not have affected the penetrance or 5-year mortality proportions, the combined weighting of the studies may have been different. We tried to assess studies that reported on nonproband/nonindex carriers, but there were limited eligible studies to assess risk of developing a second tumor and 5-year mortality for nonproband/nonindex carriers alone. Six studies included highly selected populations such as SDHB PV carriers with advanced or metastatic disease whereas a typical group of SDHB PV carriers undergoing surveillance often included asymptomatic or nonproband/index carriers. Therefore, while this was the first meta-analysis to assess risk of developing a second tumor and 5-year mortality, we acknowledge a risk of ascertainment bias as we included proband/index cases in these analyses.
Limitations to this study included the potential that search terms missed articles that were not indexed under those terms or if they were published in sources not included in the search. To counter this possibility, we also included articles identified in references. Similarly, there was the possibility of language bias; while our search was not limited to the English language, it transpired the articles selected for eligibility were all in English. Inclusion and exclusion criteria used in the review were narrow, but we felt this was appropriate given we were interested in outcomes of this specific cohort.
Surveillance for SDHB PV carriers aims to detect PPGLs before metastasis has occurred, to facilitate surgical cure (3). Our meta-analysis has potential implications for surveillance approaches among SDHB PV carriers. We found that penetrance appears to increase linearly across the lifespan up to and including age 80 years, suggesting that there is utility in ongoing screening up to age 80 years. Current guidelines recommend less screening after age 70 years (3). SDHB PV carriers with a history of tumors (either proband/index or nonproband/nonindex carriers) have a 24% risk of a second tumor, so surveillance must continue even following surgical excision of the primary tumor. The frequency of such surveillance requires further research. Moreover, the risk of metastatic progression for nonproband/nonindex carriers is 9%, which although lower than previously estimated for index cases (4) still highlights the need for careful follow-up of these cases. Apart from tumor size (5, 24, 27, 31), risk factors for metastatic progression are not currently well known. As risk factors for metastatic progression are identified in the future, health-care professionals may wish to consider more frequent surveillance for patients with these risk factors. Finally, our meta-analysis confirms a poor prognosis for SDHB PV carriers once metastatic disease has occurred, with a 5-year mortality of 18%.
Conclusion
This review has clinical relevance for genetic counseling and surveillance of all SDHB PV carriers. Nonproband/nonindex carriers have a 4% chance of having developed PPGL by age 20 years, rising to 35% by age 80 years. For carriers who develop PPGL, there is a 9% chance of metastatic progression. Since SDHB PV carriers who have developed one tumor (probands and nonproband/nonindex cases combined) have a 24% chance of developing a second tumor, follow-up surveillance is important even when the first tumor is surgically removed. In the setting of a lifelong risk of developing PPGLs and subsequent risks of metastatic progression, developing a second tumor and mortality from disease, our review supports lifelong surveillance as an important recommendation for all centers, as advocated by clinical practice guidelines (3).
Abbreviations
- HNPGL
head or neck paraganglioma
- PV
pathogenic variant
- PPGL
pheochromocytomas and paraganglioma
- SDHB
succinate dehydrogenase type B
- TAPPGL
thoracoabdominal paragangliomas and pheochromocytoma
- VUS
variants of uncertain significance
Contributor Information
Dahlia F Davidoff, Cancer Genetics Laboratory, Kolling Institute, Royal North Shore Hospital, St Leonards, NSW 2065, Australia; Faculty of Medicine and Health, University of Sydney, Northern Sydney (Arabanoo) Precinct, St Leonards, NSW 2065, Australia; Department of Endocrinology, Royal North Shore Hospital, St Leonards, NSW 2065, Australia.
Richard De Abreu Lourenco, Centre for Health Economics Research and Evaluation, University of Technology Sydney, Haymarket, Sydney 2007, Australia.
Venessa H M Tsang, Cancer Genetics Laboratory, Kolling Institute, Royal North Shore Hospital, St Leonards, NSW 2065, Australia; Faculty of Medicine and Health, University of Sydney, Northern Sydney (Arabanoo) Precinct, St Leonards, NSW 2065, Australia; Department of Endocrinology, Royal North Shore Hospital, St Leonards, NSW 2065, Australia.
Diana E Benn, Cancer Genetics Laboratory, Kolling Institute, Royal North Shore Hospital, St Leonards, NSW 2065, Australia; Faculty of Medicine and Health, University of Sydney, Northern Sydney (Arabanoo) Precinct, St Leonards, NSW 2065, Australia.
Roderick J Clifton-Bligh, Cancer Genetics Laboratory, Kolling Institute, Royal North Shore Hospital, St Leonards, NSW 2065, Australia; Faculty of Medicine and Health, University of Sydney, Northern Sydney (Arabanoo) Precinct, St Leonards, NSW 2065, Australia; Department of Endocrinology, Royal North Shore Hospital, St Leonards, NSW 2065, Australia.
Funding
D.F.D. is supported by the RACP Foundation (No. 2022RES00038). R.C.B. receives funding from the Hillcrest Foundation (No. IPAP2021/0339).
Author Contributions
D.F.D designed the study, acquired the data, performed data analyses, and drafted/approved all versions of the manuscript. R.C.B. and R.D.A.L. designed the study, aided with interpretation of data, and edited/approved the manuscript. D.B. and V.H.M.T. aided with interpretation of data and edited/approved the manuscript.
Disclosures
The authors have nothing to declare.
Data Availability
Some or all data sets generated during and/or analyzed during the current study are available from the corresponding author on request.
References
- 1. Neumann HPH, Young WF, Eng C. Pheochromocytoma and paraganglioma. N Engl J Med. 2019;381(6):552‐565. [DOI] [PubMed] [Google Scholar]
- 2. Lloyd RV, Osamura RY, Klöppel G, Rosai J, eds. WHO Classification of Tumours of Endocrine Organs. Vol 10. 4th ed. WHO/IARC Classification of Tumours; 2017. [Google Scholar]
- 3. Amar L, Pacak K, Steichen O, et al. International consensus on initial screening and follow-up of asymptomatic SDHx mutation carriers. Nat Rev Endocrinol. 2021;17(7):435‐444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bayley JP, Bausch B, Jansen JC, et al. SDHB variant type impacts phenotype and malignancy in pheochromocytoma–paraganglioma. J Med Genet. 2023;60(1):25‐32. [DOI] [PubMed] [Google Scholar]
- 5. Davidoff DF, Benn DE, Field M, et al. Surveillance improves outcomes for carriers of SDHB pathogenic variants: a multicenter study. J Clin Endocrinol Metabol. 2022;107(5):e1907‐e1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Choi YM, Lim J, Jeon MJ, et al. Mutation profile of aggressive pheochromocytoma and paraganglioma with comparison of tcga data. Cancers (Basel). 2021;13(10):2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Crona J, Lamarca A, Ghosal S, Welin S, Skogseid B, Pacak K. Genotype-phenotype correlations in pheochromocytoma and paraganglioma: a systematic review and individual patient meta-analysis. Endocr Relat Cancer. 2019;26(5):539‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hamidi O, Young WF, Gruber L, et al. Outcomes of patients with metastatic phaeochromocytoma and paraganglioma: a systematic review and meta-analysis. Clin Endocrinol (Oxf). 2017;87(5):440‐450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee H, Jeong S, Yu Y, et al. Risk of metastatic pheochromocytoma and paraganglioma in SDHx mutation carriers: a systematic review and updated meta-analysis. J Med Genet. 2020;57(4):217‐225. [DOI] [PubMed] [Google Scholar]
- 10. van Hulsteijn LT, Dekkers OM, Hes FJ, Smit JWA, Corssmit EPM. Risk of malignant paraganglioma in SDHB-mutation and SDHD-mutation carriers: a systematic review and meta-analysis. J Med Genet. 2012;49(12):768‐776. [DOI] [PubMed] [Google Scholar]
- 11. Lenders JW, Duh QY, Eisenhofer G, et al. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metabol. 2014;99(6):1915‐1942. [DOI] [PubMed] [Google Scholar]
- 12. Amar L, Baudin E, Burnichon N, et al. Succinate dehydrogenase B gene mutations predict survival in patients with malignant pheochromocytomas or paragangliomas. J Clin Endocrinol Metabol. 2007;92(10):3822‐3828. [DOI] [PubMed] [Google Scholar]
- 13. Tufton N, Shapiro L, Srirangalingam U, et al. Outcomes of annual surveillance imaging in an adult and paediatric cohort of succinate dehydrogenase B mutation carriers. Clin Endocrinol (Oxf). 2017;86(2):286‐296. [DOI] [PubMed] [Google Scholar]
- 14. Davidoff DF, De Abreu Lourenco R, Tsang VH, Benn DE, Clifton-Bligh RJ. Data from: Supplemental Material Outcomes of SDHB pathogenic variant carriers a systematic review and meta-analysis [Data set]. Zenodo; 2023. 10.5281/zenodo.8423607. [DOI] [PMC free article] [PubMed]
- 15. Munnangi S, Boktor SW. Epidemiology of Study Design. StatPearls Publishing; 2023. [PubMed] [Google Scholar]
- 16. Andrews KA, Ascher DB, Pires DEV, et al. Tumour risks and genotype-phenotype correlations associated with germline variants in succinate dehydrogenase subunit genes SDHB, SDHC and SDHD. J Med Genet. 2018;55(6):384‐394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. John Wiley & Sons, Ltd; 2009. [Google Scholar]
- 18. Rothstein HR, Sutton AJ, Borenstein M. Publication Bias in Meta-Analysis: Prevention, Assessment and Adjustments. Wiley; 2005. [Google Scholar]
- 19. Bausch B, Wellner U, Bausch D, et al. Long-term prognosis of patients with pediatric pheochromocytoma. Endocr Relat Cancer. 2014;21(1):17‐25. [DOI] [PubMed] [Google Scholar]
- 20. Daniel E, Jones R, Bull M, Newell-Price J. Rapid-sequence MRI for long-term surveillance for paraganglioma and phaeochromocytoma in patients with succinate dehydrogenase mutations. Eur J Endocrinol. 2016;175(6):561‐570. [DOI] [PubMed] [Google Scholar]
- 21. Eijkelenkamp K, Osinga TE, de Jong MM, et al. Calculating the optimal surveillance for head and neck paraganglioma in SDHB-mutation carriers. Fam Cancer. 2017;16(1):123‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jafri M, Whitworth J, Rattenberry E, et al. Evaluation of SDHB, SDHD and VHL gene susceptibility testing in the assessment of individuals with non-syndromic phaeochromocytoma, paraganglioma and head and neck paraganglioma. Clin Endocrinol (Oxf). 2013;78(6):898‐906. [DOI] [PubMed] [Google Scholar]
- 23. Jochmanova I, Wolf KI, King KS, et al. SDHB-related pheochromocytoma and paraganglioma penetrance and genotype-phenotype correlations. J Cancer Res Clin Oncol. 2017;143(8):1421‐1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jochmanova I, Abcede AMT, Guerrero RJS, et al. Clinical characteristics and outcomes of SDHB-related pheochromocytoma and paraganglioma in children and adolescents. J Cancer Res Clin Oncol. 2020;146(4):1051‐1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. King KS, Prodanov T, Kantorovich V, et al. Metastatic pheochromocytoma/paraganglioma related to primary tumor development in childhood or adolescence: significant link to SDHB mutations. J Clin Oncol. 2011;29(31):4137‐4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Niemeijer ND, Rijken JA, Eijkelenkamp K, et al. The phenotype of SDHB germline mutation carriers: a nationwide study. European journal of endocrinology. 2017;177(2):115‐125. [DOI] [PubMed] [Google Scholar]
- 27. Schovanek J, Martucci V, Wesley R, et al. The size of the primary tumor and age at initial diagnosis are independent predictors of the metastatic behavior and survival of patients with SDHB-related pheochromocytoma and paraganglioma: a retrospective cohort study. BMC cancer. 2014;14(1):523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Srirangalingam U, Walker L, Khoo B, et al. Clinical manifestations of familial paraganglioma and phaeochromocytomas in succinate dehydrogenase B (SDH-B) gene mutation carriers. Clin Endocrinol (Oxf). 2008;69(4):587‐596. [DOI] [PubMed] [Google Scholar]
- 29. Turkova H, Prodanov T, Maly M, et al. Characteristics and outcomes of metastatic SDHB and sporadic pheochromocytoma/paraganglioma: an national institutes of health study. Endocr Pract. 2016;22(3):302‐314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. White G, Velusamy A, Anandappa S, et al. Tumour detection and outcomes of surveillance screening in SDHB and SDHD pathogenic variant carriers. Endocr Connect. 2022;11(2):e210602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hescot S, Curras-Freixes M, Deutschbein T, et al. Prognosis of malignant pheochromocytoma and paraganglioma (MAPP-PronO study): a European network for the study of adrenal tumors retrospective study. The Journal of Clinical Endocrinology & Metabolism. 2019;104(6):2367‐2374. [DOI] [PubMed] [Google Scholar]
- 32. Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11(2):193‐206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Davidoff DF, De Abreu Lourenco R, Tsang VH, Benn DE, Clifton-Bligh RJ. Data from: Supplemental Material Outcomes of SDHB pathogenic variant carriers a systematic review and meta-analysis [Data set]. Zenodo; 2023. 10.5281/zenodo.8423607. [DOI] [PMC free article] [PubMed]
Data Availability Statement
Some or all data sets generated during and/or analyzed during the current study are available from the corresponding author on request.