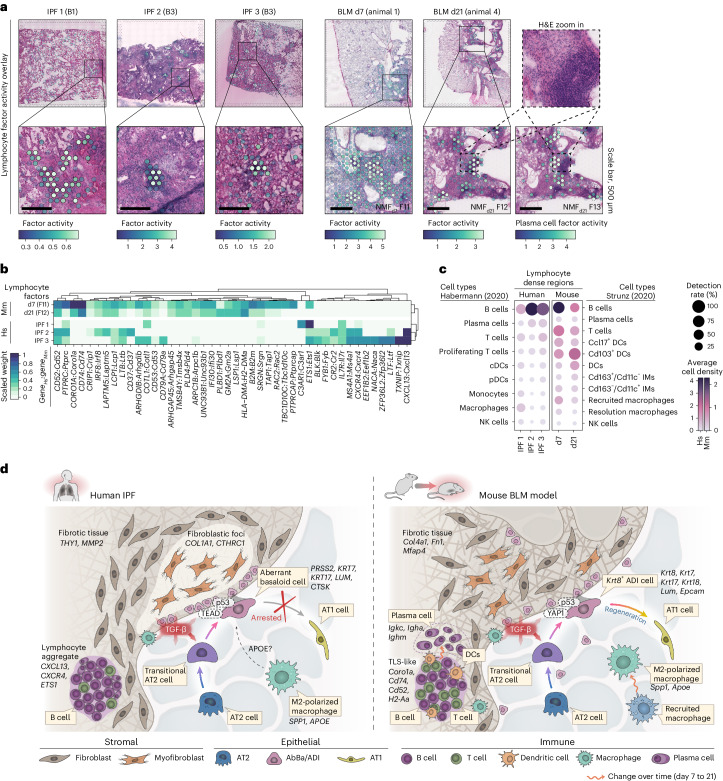
Fig. 6. Immune cell signatures and comparative overview of the fibrotic niche in IPF and the BLM mouse model.
a, Spatial visualization of NMF factors overlapping dense lymphocyte or immune cell aggregates in selected human and mouse samples. Scale bars, 500 µm. Imaged at 20× magnification. b, Heat map displays the top contributing factor genes across condition, filtered to show genes with a summed scaled weight above 0.5 across the groups. c, Dot plot with inferred cell-type densities, for selected immune cell types from the ‘Habermann5’ and ‘Strunz11’ datasets, in the most active spots of the selected human and mouse factors. d, Schematic summary of the fibrotic niche in human IPF lungs and in mouse BLM-injured lungs, illustrating the proposed cellular interplay within the fibrotic lungs. A key distinction between IPF and the BLM mouse model was centered around the diverging regenerative properties of the IPF-associated AbBa cells versus the mouse Krt8+ ADI cells. While both populations exhibit signs of senescence (p53), the mouse ADI state appears to maintain a functional balance that still prompts it to differentiate into AT1 cells. TGF-β and Wnt-related (TEAD, YAP1) signaling pathways were central within the fibrotic niche, and the presence of immune cells in proximity to, or within, the severely remodeled tissue implies active fibrogenic modulatory roles. Pro-fibrotic M2-polarized (‘resolution’) macrophages with similar gene signatures, expressing SPP1 (Spp1) and APOE (Apoe) were detected in both human IPF and mouse BLM-injured lungs. In contrast to human IPF AbBa regions, a predicted negative APOE upstream signaling was identified in mouse ADI regions. In mouse, the recruited proinflammatory macrophages seen at the early time point post BLM-installation were absent by d21. Establishment of plasma cells adjacent to TLS-like areas in the BLM-injured mice occurred at the later time point. pDC/cDCs, plasmacytoid/classical dendritic cells; IMs, interstitial macrophages; NK cells, natural killer cells; TLS, tertiary lymphoid structure.