Abstract
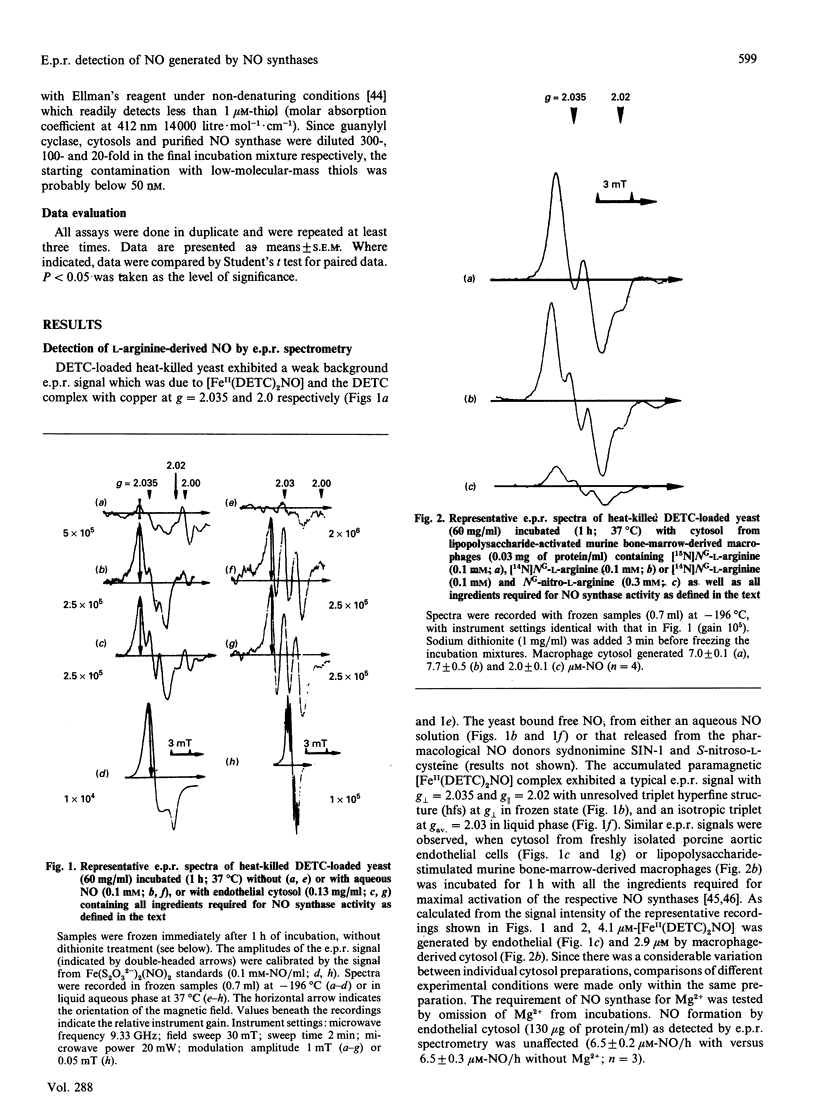
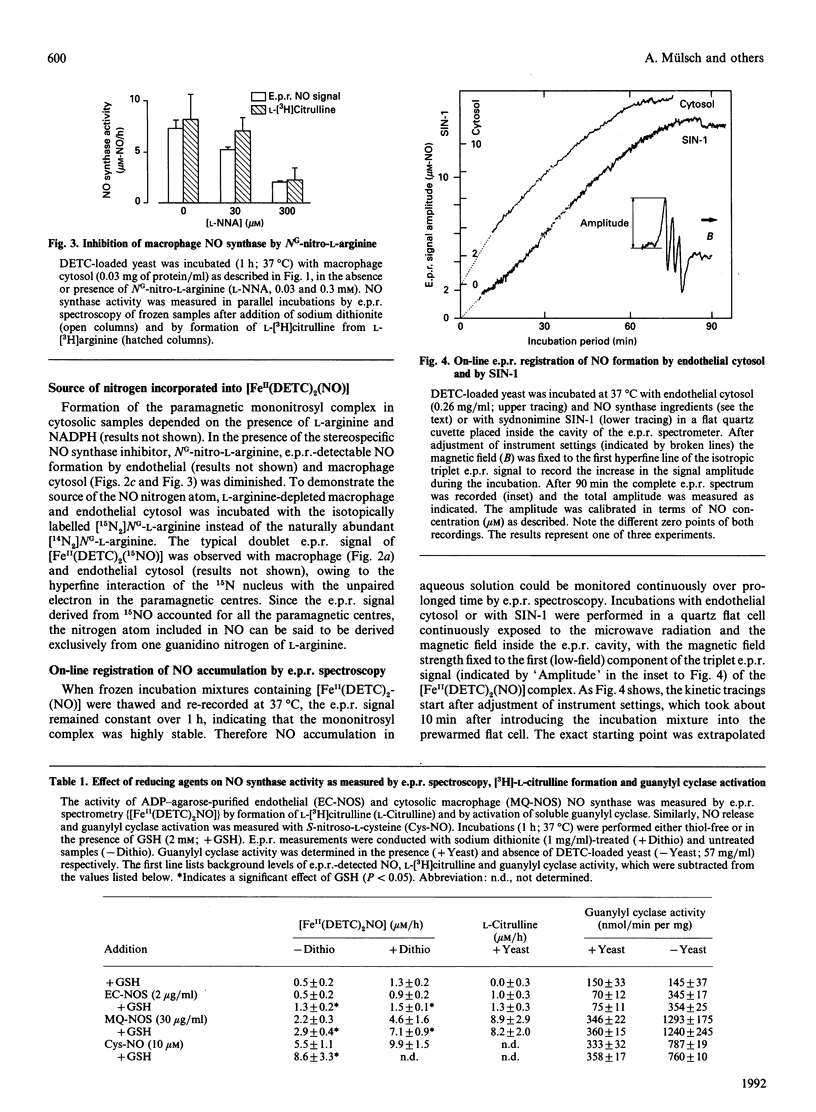
We have assessed the stoichiometry of the nitric oxide (NO) synthase reaction by using a novel e.p.r. technique. NO generated by crude and partially purified NO synthase from endothelial cells and Escherichia coli-lipopolysaccharide-activated macrophages was trapped by a ferrous diethyldithiocarbamate complex dispersed in yeast. The paramagnetic ferrous mononitrosyl dithiocarbamate complex formed exhibited a characteristic e.p.r. signal at g perpendicular = 2.035 and g parallel = 2.02 with a triplet hyperfine structure (hfs) at g perpendicular. NO, 3-morpholinosydnonimine and S-nitroso-L-cysteine, but not nitrite or hydroxylamine, generated a similar e.p.r. signal. NO generated by NO synthase and by SIN-1 accumulated at a constant rate for 1 h, as measured by continuous e.p.r. registration at 37 degrees C. The formation of e.p.r.-detectable NO by NO synthases was inhibited by NG-nitro-L-arginine. Incubation with [15N]NG-L-arginine caused an e.p.r. signal with doublet hfs, indicating that the nitrosyl nitrogen derived exclusively from the guanidino nitrogen. The amount of NO generated by NO synthase as measured by e.p.r. technique was compared with formation of L-[3H]citrulline from L-[3H]arginine. NO and L-citrulline were detected at a 1:1 ratio with both NO synthase preparations. GSH and thiol depletion did not significantly affect NO synthase activity, excluding S-nitrosothiols as intermediates in the NO synthase reaction. We conclude that NO fully accounts for the immediate oxygenated nitrogen species derived from the enzymic oxygenation of L-arginine.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arroyo C. M., Forray C., el-Fakahany E. E., Rosen G. M. Receptor-mediated generation of an EDRF-like intermediate in a neuronal cell line detected by spin trapping techniques. Biochem Biophys Res Commun. 1990 Aug 16;170(3):1177–1183. doi: 10.1016/0006-291x(90)90517-q. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Nitric oxide mediates glutamate-linked enhancement of cGMP levels in the cerebellum. Proc Natl Acad Sci U S A. 1989 Nov;86(22):9030–9033. doi: 10.1073/pnas.86.22.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse R., Lückhoff A., Bassenge E. Endothelium-derived relaxant factor inhibits platelet activation. Naunyn Schmiedebergs Arch Pharmacol. 1987 Nov;336(5):566–571. doi: 10.1007/BF00169315. [DOI] [PubMed] [Google Scholar]
- Doyle M. P., Hoekstra J. W. Oxidation of nitrogen oxides by bound dioxygen in hemoproteins. J Inorg Biochem. 1981 Jul;14(4):351–358. doi: 10.1016/s0162-0134(00)80291-3. [DOI] [PubMed] [Google Scholar]
- Giovanelli J., Campos K. L., Kaufman S. Tetrahydrobiopterin, a cofactor for rat cerebellar nitric oxide synthase, does not function as a reactant in the oxygenation of arginine. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7091–7095. doi: 10.1073/pnas.88.16.7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover J. H. Chemiluminescence in gas analysis and Flame-emission spectrometry. Analyst. 1975 Jul;100(1192):449–464. doi: 10.1039/an9750000449. [DOI] [PubMed] [Google Scholar]
- Goretski J., Hollocher T. C. The kinetic and isotopic competence of nitric oxide as an intermediate in denitrification. J Biol Chem. 1990 Jan 15;265(2):889–895. [PubMed] [Google Scholar]
- Green L. C., Ruiz de Luzuriaga K., Wagner D. A., Rand W., Istfan N., Young V. R., Tannenbaum S. R. Nitrate biosynthesis in man. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7764–7768. doi: 10.1073/pnas.78.12.7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauschildt S., Lückhoff A., Mülsch A., Kohler J., Bessler W., Busse R. Induction and activity of NO synthase in bone-marrow-derived macrophages are independent of Ca2+. Biochem J. 1990 Sep 1;270(2):351–356. doi: 10.1042/bj2700351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J. Nitric oxide. A novel signal transduction mechanism for transcellular communication. Hypertension. 1990 Nov;16(5):477–483. doi: 10.1161/01.hyp.16.5.477. [DOI] [PubMed] [Google Scholar]
- Iyengar R., Stuehr D. J., Marletta M. A. Macrophage synthesis of nitrite, nitrate, and N-nitrosamines: precursors and role of the respiratory burst. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6369–6373. doi: 10.1073/pnas.84.18.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelm M., Schrader J. Control of coronary vascular tone by nitric oxide. Circ Res. 1990 Jun;66(6):1561–1575. doi: 10.1161/01.res.66.6.1561. [DOI] [PubMed] [Google Scholar]
- Kondo K., Mitchell J. A., de Nucci G., Vane J. R. Simultaneous measurement of endothelium-derived relaxing factor by bioassay and guanylate cyclase stimulation. Br J Pharmacol. 1989 Oct;98(2):630–636. doi: 10.1111/j.1476-5381.1989.tb12637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka H., Tyuma I. Mechanism of autocatalytic oxidation of oxyhemoglobin by nitrite. Environ Health Perspect. 1987 Aug;73:147–151. doi: 10.1289/ehp.8773147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon N. S., Nathan C. F., Stuehr D. J. Reduced biopterin as a cofactor in the generation of nitrogen oxides by murine macrophages. J Biol Chem. 1989 Dec 5;264(34):20496–20501. [PubMed] [Google Scholar]
- Lancaster J. R., Jr, Hibbs J. B., Jr EPR demonstration of iron-nitrosyl complex formation by cytotoxic activated macrophages. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1223–1227. doi: 10.1073/pnas.87.3.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer B., John M., Heinzel B., Werner E. R., Wachter H., Schultz G., Böhme E. Brain nitric oxide synthase is a biopterin- and flavin-containing multi-functional oxido-reductase. FEBS Lett. 1991 Aug 19;288(1-2):187–191. doi: 10.1016/0014-5793(91)81031-3. [DOI] [PubMed] [Google Scholar]
- Menon N. K., Patricza J., Binder T., Bing R. J. Reduction of biological effluents in purge and trap micro reaction vessels and detection of endothelium-derived nitric oxide (edno) by chemiluminescence. J Mol Cell Cardiol. 1991 Apr;23(4):389–393. doi: 10.1016/0022-2828(91)90162-f. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Mordvintcev P., Mülsch A., Busse R., Vanin A. On-line detection of nitric oxide formation in liquid aqueous phase by electron paramagnetic resonance spectroscopy. Anal Biochem. 1991 Nov 15;199(1):142–146. doi: 10.1016/0003-2697(91)90282-x. [DOI] [PubMed] [Google Scholar]
- Myers P. R., Minor R. L., Jr, Guerra R., Jr, Bates J. N., Harrison D. G. Vasorelaxant properties of the endothelium-derived relaxing factor more closely resemble S-nitrosocysteine than nitric oxide. Nature. 1990 May 10;345(6271):161–163. doi: 10.1038/345161a0. [DOI] [PubMed] [Google Scholar]
- Mülsch A., Bassenge E., Busse R. Nitric oxide synthesis in endothelial cytosol: evidence for a calcium-dependent and a calcium-independent mechanism. Naunyn Schmiedebergs Arch Pharmacol. 1989 Dec;340(6 Pt 2):767–770. doi: 10.1007/BF00169688. [DOI] [PubMed] [Google Scholar]
- Mülsch A., Böhme E., Busse R. Stimulation of soluble guanylate cyclase by endothelium-derived relaxing factor from cultured endothelial cells. Eur J Pharmacol. 1987 Mar 17;135(2):247–250. doi: 10.1016/0014-2999(87)90620-0. [DOI] [PubMed] [Google Scholar]
- Mülsch A., Gerzer R. Purification of heme-containing soluble guanylyl cyclase. Methods Enzymol. 1991;195:377–383. doi: 10.1016/0076-6879(91)95183-k. [DOI] [PubMed] [Google Scholar]
- Mülsch A., Mordvintcev P., Vanin A. F., Busse R. The potent vasodilating and guanylyl cyclase activating dinitrosyl-iron(II) complex is stored in a protein-bound form in vascular tissue and is released by thiols. FEBS Lett. 1991 Dec 9;294(3):252–256. doi: 10.1016/0014-5793(91)81441-a. [DOI] [PubMed] [Google Scholar]
- Niroomand F., Mülsch A., Böhme E. Thiol-independent stimulation of soluble guanylate cyclase by NO-containing compounds. Biochem Pharmacol. 1991 Jun 1;41(11):1777–1779. doi: 10.1016/0006-2952(91)90185-8. [DOI] [PubMed] [Google Scholar]
- Pai T. G., Payne W. J., LeGall J. Use of a chemiluminescence detector for quantitation of nitric oxide produced in assays of denitrifying enzymes. Anal Biochem. 1987 Oct;166(1):150–157. doi: 10.1016/0003-2697(87)90557-4. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Pellat C., Henry Y., Drapier J. C. IFN-gamma-activated macrophages: detection by electron paramagnetic resonance of complexes between L-arginine-derived nitric oxide and non-heme iron proteins. Biochem Biophys Res Commun. 1990 Jan 15;166(1):119–125. doi: 10.1016/0006-291x(90)91919-j. [DOI] [PubMed] [Google Scholar]
- Prónai L., Ichimori K., Nozaki H., Nakazawa H., Okino H., Carmichael A. J., Arroyo C. M. Investigation of the existence and biological role of L-arginine/nitric oxide pathway in human platelets by spin-trapping/EPR studies. Eur J Biochem. 1991 Dec 18;202(3):923–930. doi: 10.1111/j.1432-1033.1991.tb16452.x. [DOI] [PubMed] [Google Scholar]
- Riddles P. W., Blakeley R. L., Zerner B. Reassessment of Ellman's reagent. Methods Enzymol. 1983;91:49–60. doi: 10.1016/s0076-6879(83)91010-8. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H., Zernikow B., Baeblich S., Böhme E. Basal and stimulated formation and release of L-arginine-derived nitrogen oxides from cultured endothelial cells. J Pharmacol Exp Ther. 1990 Aug;254(2):591–597. [PubMed] [Google Scholar]
- Stuehr D. J., Gross S. S., Sakuma I., Levi R., Nathan C. F. Activated murine macrophages secrete a metabolite of arginine with the bioactivity of endothelium-derived relaxing factor and the chemical reactivity of nitric oxide. J Exp Med. 1989 Mar 1;169(3):1011–1020. doi: 10.1084/jem.169.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuehr D. J., Kwon N. S., Nathan C. F., Griffith O. W., Feldman P. L., Wiseman J. N omega-hydroxy-L-arginine is an intermediate in the biosynthesis of nitric oxide from L-arginine. J Biol Chem. 1991 Apr 5;266(10):6259–6263. [PubMed] [Google Scholar]
- Thomas G., Ramwell P. W. Vascular relaxation mediated by hydroxylamines and oximes: their conversion to nitrites and mechanism of endothelium dependent vascular relaxation. Biochem Biophys Res Commun. 1989 Oct 31;164(2):889–893. doi: 10.1016/0006-291x(89)91542-8. [DOI] [PubMed] [Google Scholar]
- Tracey W. R., Linden J., Peach M. J., Johns R. A. Comparison of spectrophotometric and biological assays for nitric oxide (NO) and endothelium-derived relaxing factor (EDRF): nonspecificity of the diazotization reaction for NO and failure to detect EDRF. J Pharmacol Exp Ther. 1990 Mar;252(3):922–928. [PubMed] [Google Scholar]
- Vanin A. F. Endothelium-derived relaxing factor is a nitrosyl iron complex with thiol ligands. FEBS Lett. 1991 Sep 2;289(1):1–3. doi: 10.1016/0014-5793(91)80894-9. [DOI] [PubMed] [Google Scholar]


