Abstract
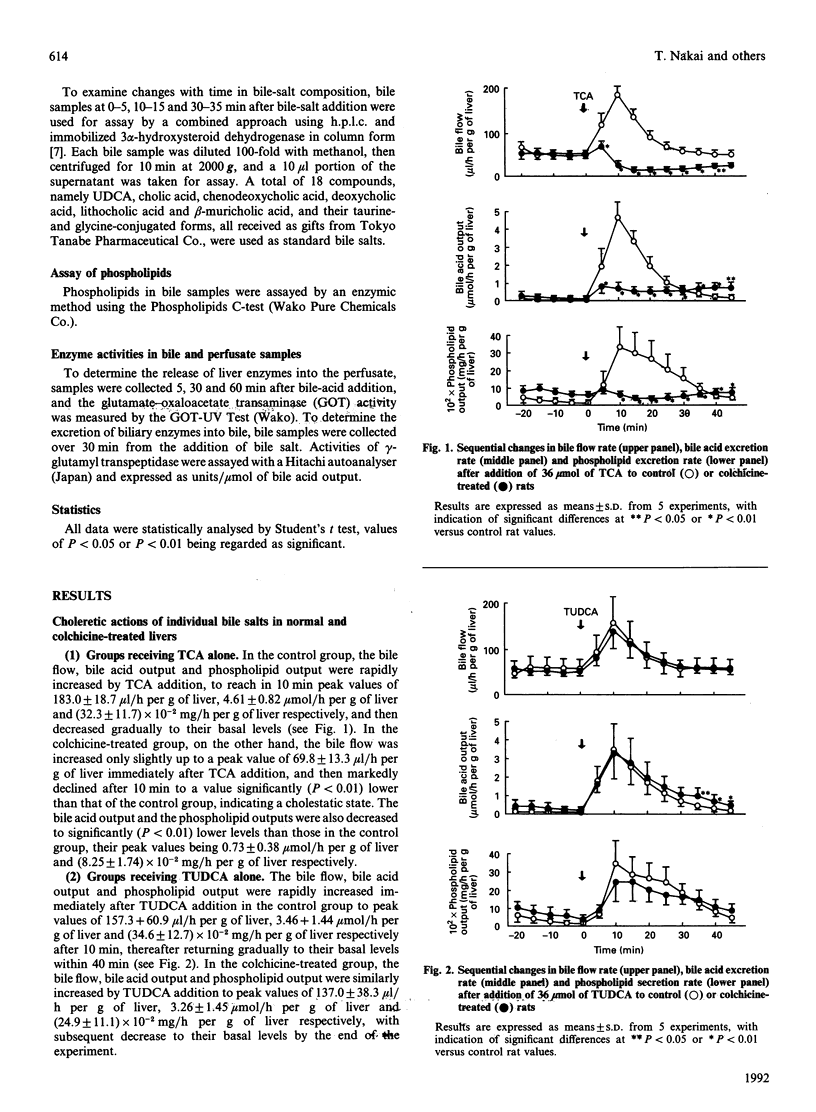
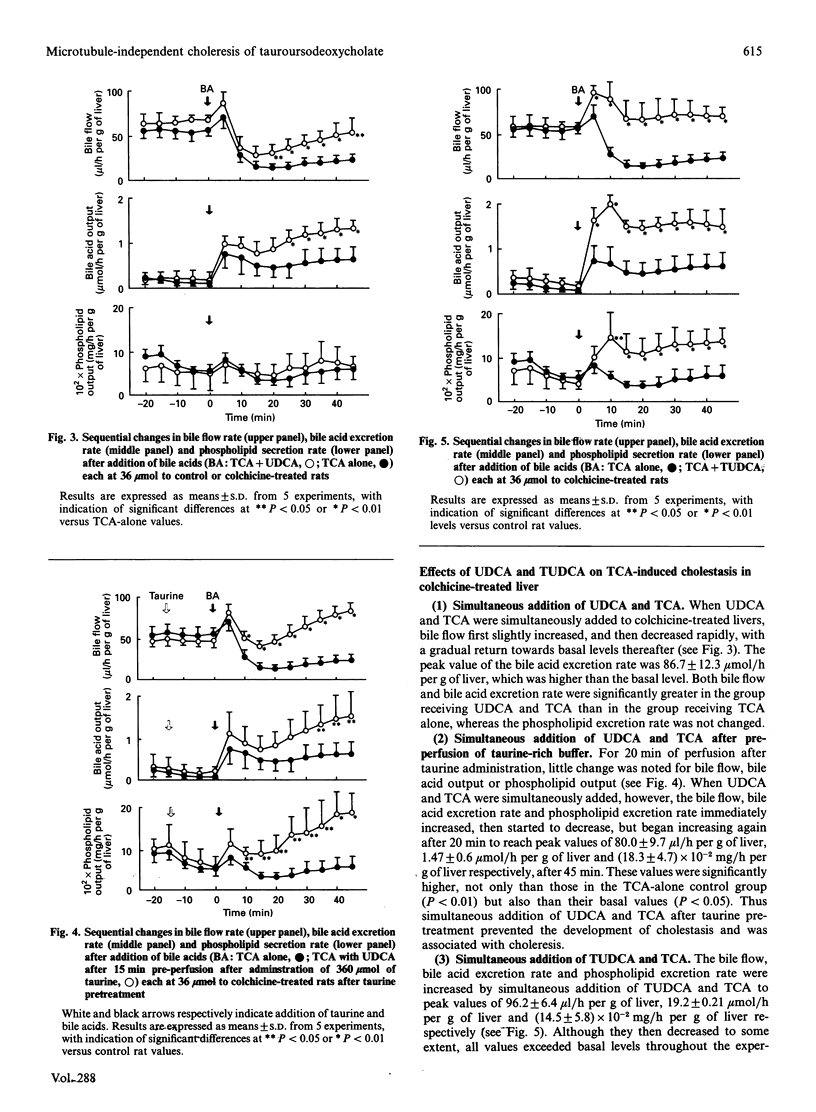
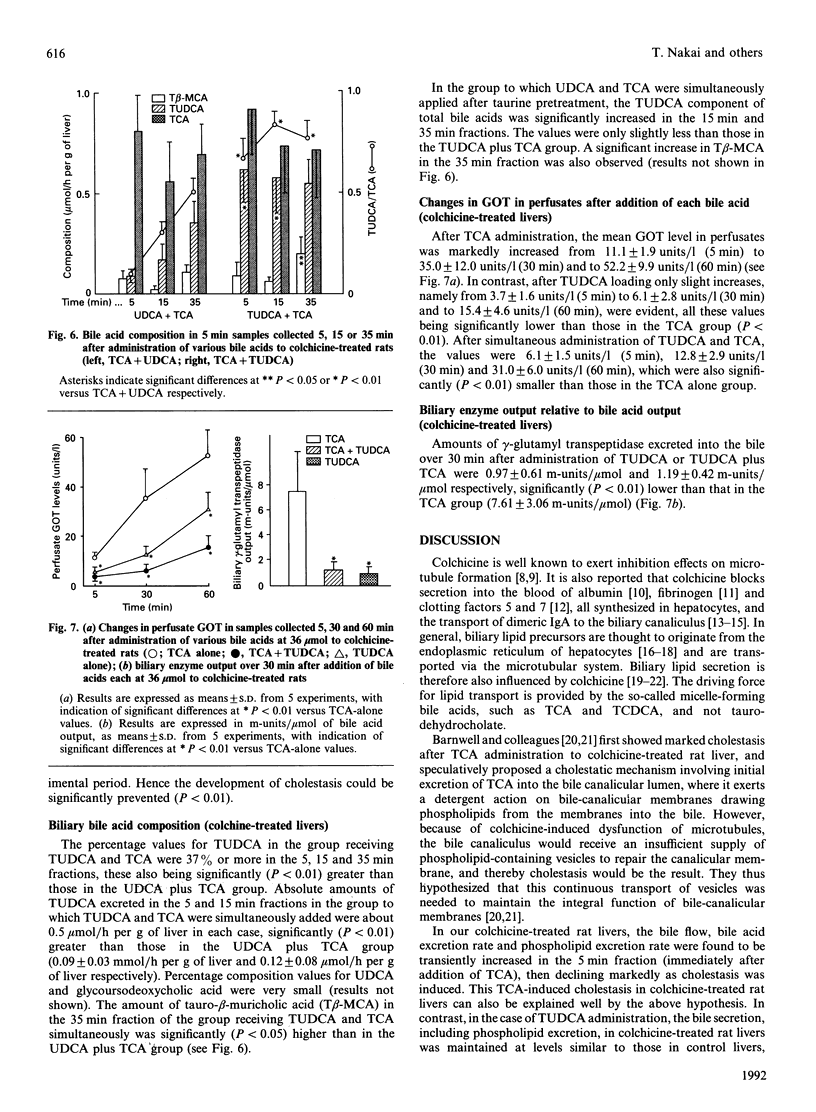
In order to cast light on the anti-cholestatic and cytoprotective properties of ursodeoxycholic acid (UDCA), intrahepatic transport and secretion of bile salts and biliary phospholipids were investigated by using isolated perfused livers from colchicine-pretreated rats. Administration of taurocholic acid (TCA) after colchicine pretreatment induced marked cholestasis. Tauroursodeoxycholic acid (TUDCA) treatment, in contrast, was associated with maintenance of bile flow, with excretion rates of bile acids and phospholipids similar to those in control animals. Furthermore, TCA-induced cholestasis in colchicine-treated rat livers was clearly decreased by co-administration of TUDCA. Although simultaneous addition of UDCA also showed slight improvement, with or without taurine pre-treatment, biliary bile-salt analysis also showed that cholestasis was markedly remitted as the excretion of taurine-conjugated UDCA was increased. The results suggest that the cytoprotective and anti-cholestatic effects of TUDCA may be linked to action at the intrahepatocyte level, represented by mild detergent effects on organelle lipids and preservation of intracellular transport even under microtubule-dysfunctional conditions. In addition, it was indicated that cytoprotective effects of UDCA may also be exerted after its conjugation with taurine inside hepatocytes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong M. J., Carey M. C. The hydrophobic-hydrophilic balance of bile salts. Inverse correlation between reverse-phase high performance liquid chromatographic mobilities and micellar cholesterol-solubilizing capacities. J Lipid Res. 1982 Jan;23(1):70–80. [PubMed] [Google Scholar]
- Balint J. A., Beeler D. A., Treble D. H., Spitzer H. L. Studies in the biosynthesis of hepatic and biliary lecithins. J Lipid Res. 1967 Sep;8(5):486–493. [PubMed] [Google Scholar]
- Barnwell S. G., Coleman R. Abnormal secretion of proteins into bile from colchicine-treated isolated perfused rat livers. Biochem J. 1983 Nov 15;216(2):409–414. doi: 10.1042/bj2160409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnwell S. G., Lowe P. J., Coleman R. The effects of colchicine on secretion into bile of bile salts, phospholipids, cholesterol and plasma membrane enzymes: bile salts are secreted unaccompanied by phospholipids and cholesterol. Biochem J. 1984 Jun 15;220(3):723–731. doi: 10.1042/bj2200723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellentani S., Hardison W. G., Marchegiano P., Zanasi G., Manenti F. Bile acid inhibition of taurocholate uptake by rat hepatocytes: role of OH groups. Am J Physiol. 1987 Mar;252(3 Pt 1):G339–G344. doi: 10.1152/ajpgi.1987.252.3.G339. [DOI] [PubMed] [Google Scholar]
- Crawford J. M., Berken C. A., Gollan J. L. Role of the hepatocyte microtubular system in the excretion of bile salts and biliary lipid: implications for intracellular vesicular transport. J Lipid Res. 1988 Feb;29(2):144–156. [PubMed] [Google Scholar]
- Feldmann G., Maurice M., Sapin C., Benhamou J. P. Inhibition by colchicine of fibrinogen translocation in hepatocytes. J Cell Biol. 1975 Oct;67(1):237–243. doi: 10.1083/jcb.67.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey P. P., Lembra L., Coleman R. Effects of colchicine and vinblastine on output of proteins into bile. Biochem J. 1982 Oct 15;208(1):153–157. doi: 10.1042/bj2080153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory D. H., Vlahcevic Z. R., Prugh M. F., Swell L. Mechanism of secretion of biliary lipids: role of a microtubular system in hepatocellular transport of biliary lipids in the rat. Gastroenterology. 1978 Jan;74(1):93–100. [PubMed] [Google Scholar]
- Gregory D. H., Vlahcevic Z. R., Schatzki P., Swell L. Mechanism of secretion of biliary lipids. I. Role of bile canalicular and microsomal membranes in the synthesis and transport of biliary lecithin and cholesterol. J Clin Invest. 1975 Jan;55(1):105–114. doi: 10.1172/JCI107900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuman D. M., Mills A. S., McCall J., Hylemon P. B., Pandak W. M., Vlahcevic Z. R. Conjugates of ursodeoxycholate protect against cholestasis and hepatocellular necrosis caused by more hydrophobic bile salts. In vivo studies in the rat. Gastroenterology. 1991 Jan;100(1):203–211. doi: 10.1016/0016-5085(91)90602-h. [DOI] [PubMed] [Google Scholar]
- Hofmann A. F., Popper H. Ursodeoxycholic acid for primary biliary cirrhosis. Lancet. 1987 Aug 15;2(8555):398–399. doi: 10.1016/s0140-6736(87)92421-4. [DOI] [PubMed] [Google Scholar]
- Kanai S., Ohta M., Kitani K., Sato Y. Tauro beta-muricholate is as effective as tauroursodeoxycholate in preventing taurochenodeoxycholate-induced liver damage in the rat. Life Sci. 1990;47(26):2421–2428. doi: 10.1016/0024-3205(90)90486-b. [DOI] [PubMed] [Google Scholar]
- Kitani K., Kanai S. Tauroursodeoxycholate prevents taurocholate induced cholestasis. Life Sci. 1982 Feb 7;30(6):515–523. doi: 10.1016/0024-3205(82)90264-8. [DOI] [PubMed] [Google Scholar]
- Kitani K., Ohta M., Kanai S. Tauroursodeoxycholate prevents biliary protein excretion induced by other bile salts in the rat. Am J Physiol. 1985 Apr;248(4 Pt 1):G407–G417. doi: 10.1152/ajpgi.1985.248.4.G407. [DOI] [PubMed] [Google Scholar]
- Le Marchand Y., Patzelt C., Assimacopoulos-Jeannet F., Loten E. G., Jeanrenaud B. Evidence for a role of the microtubular system in the secretion of newly synthesized albumin and other proteins by the liver. J Clin Invest. 1974 Jun;53(6):1512–1517. doi: 10.1172/JCI107701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuschner U., Leuschner M., Sieratzki J., Kurtz W., Hübner K. Gallstone dissolution with ursodeoxycholic acid in patients with chronic active hepatitis and two years follow-up. A pilot study. Dig Dis Sci. 1985 Jul;30(7):642–649. doi: 10.1007/BF01308413. [DOI] [PubMed] [Google Scholar]
- Lowe P. J., Barnwell S. G., Coleman R. Rapid kinetic analysis of the bile-salt-dependent secretion of phospholipid, cholesterol and a plasma-membrane enzyme into bile. Biochem J. 1984 Sep 15;222(3):631–637. doi: 10.1042/bj2220631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullock B. M., Jones R. S., Peppard J., Hinton R. H. Effect of colchicine on the transfer of IgA across hepatocytes into bile in isolated perfused rat livers. FEBS Lett. 1980 Nov 3;120(2):278–282. doi: 10.1016/0014-5793(80)80316-4. [DOI] [PubMed] [Google Scholar]
- Owellen R. J., Owens A. H., Jr, Donigian D. W. The binding of vincristine, vinblastine and colchicine to tubulin. Biochem Biophys Res Commun. 1972 May 26;47(4):685–691. doi: 10.1016/0006-291x(72)90546-3. [DOI] [PubMed] [Google Scholar]
- Poupon R., Chrétien Y., Poupon R. E., Ballet F., Calmus Y., Darnis F. Is ursodeoxycholic acid an effective treatment for primary biliary cirrhosis? Lancet. 1987 Apr 11;1(8537):834–836. doi: 10.1016/s0140-6736(87)91610-2. [DOI] [PubMed] [Google Scholar]
- Reuben A., Allen R. M. Intrahepatic sources of biliary-like micelles. Biochim Biophys Acta. 1986 Mar 21;876(1):1–12. doi: 10.1016/0005-2760(86)90311-5. [DOI] [PubMed] [Google Scholar]
- Stiehl A., Raedsch R., Rudolph G. Acute effects of ursodeoxycholic and chenodeoxycholic acid on the small intestinal absorption of bile acids. Gastroenterology. 1990 Feb;98(2):424–428. doi: 10.1016/0016-5085(90)90834-n. [DOI] [PubMed] [Google Scholar]
- Tomoda T., Moriwaki H., Onishi H., Tomita E., Takai T., Muto Y., Kumada T., Okuyama S. [Therapeutic effect of ursodeoxycholic acid on sulpyrine-induced intrahepatic cholestasis. A case report]. Nihon Shokakibyo Gakkai Zasshi. 1984 Nov;81(11):2821–2825. [PubMed] [Google Scholar]
- Wilson L., Bamburg J. R., Mizel S. B., Grisham L. M., Creswell K. M. Interaction of drugs with microtubule proteins. Fed Proc. 1974 Feb;33(2):158–166. [PubMed] [Google Scholar]


