Abstract
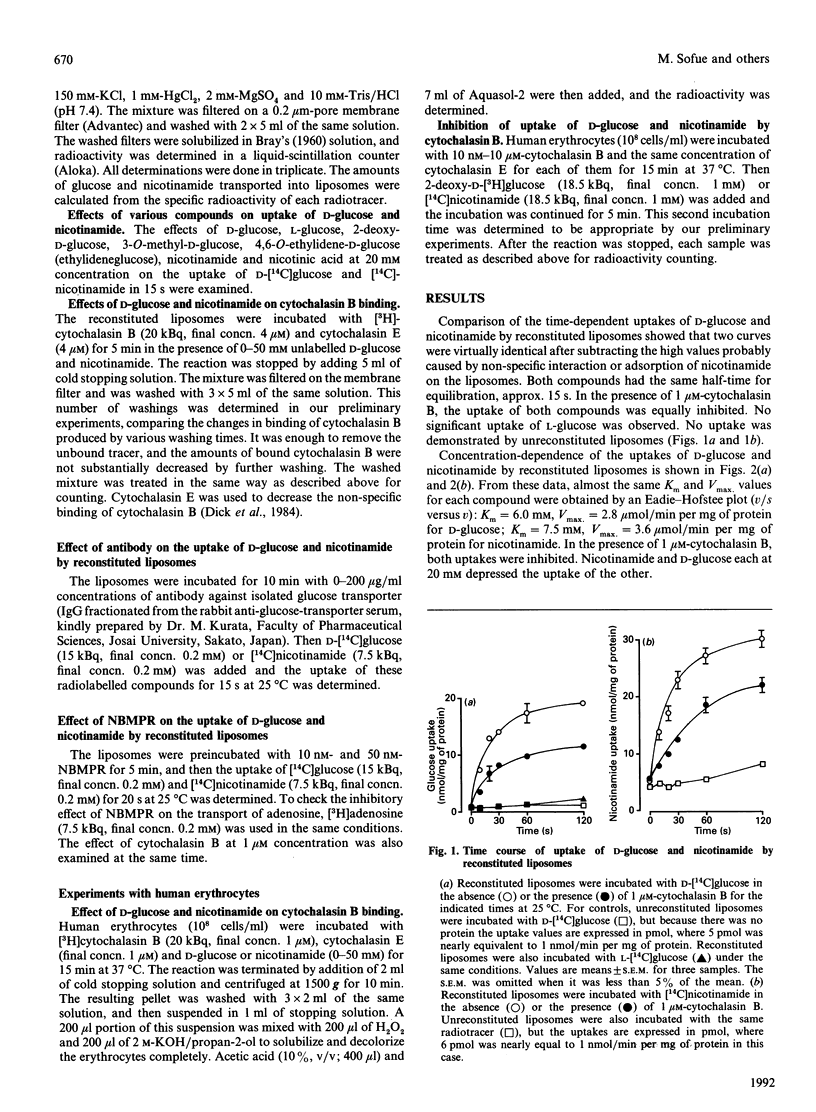
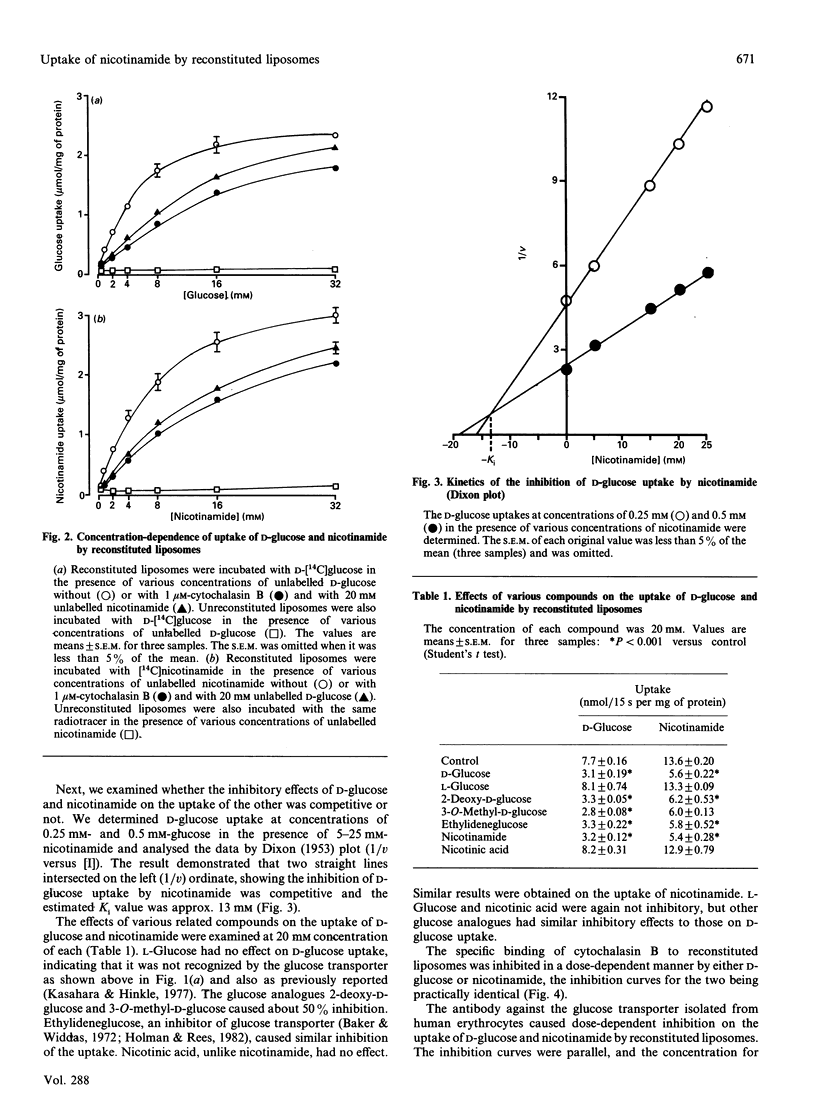
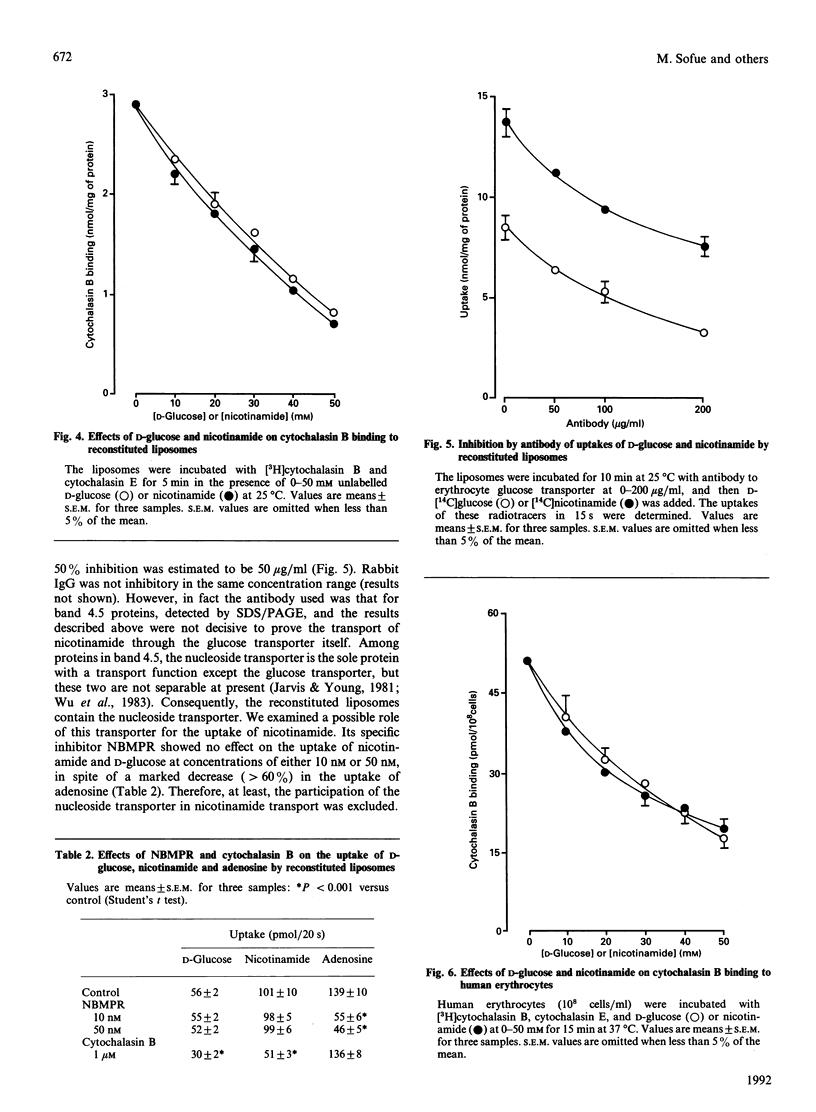
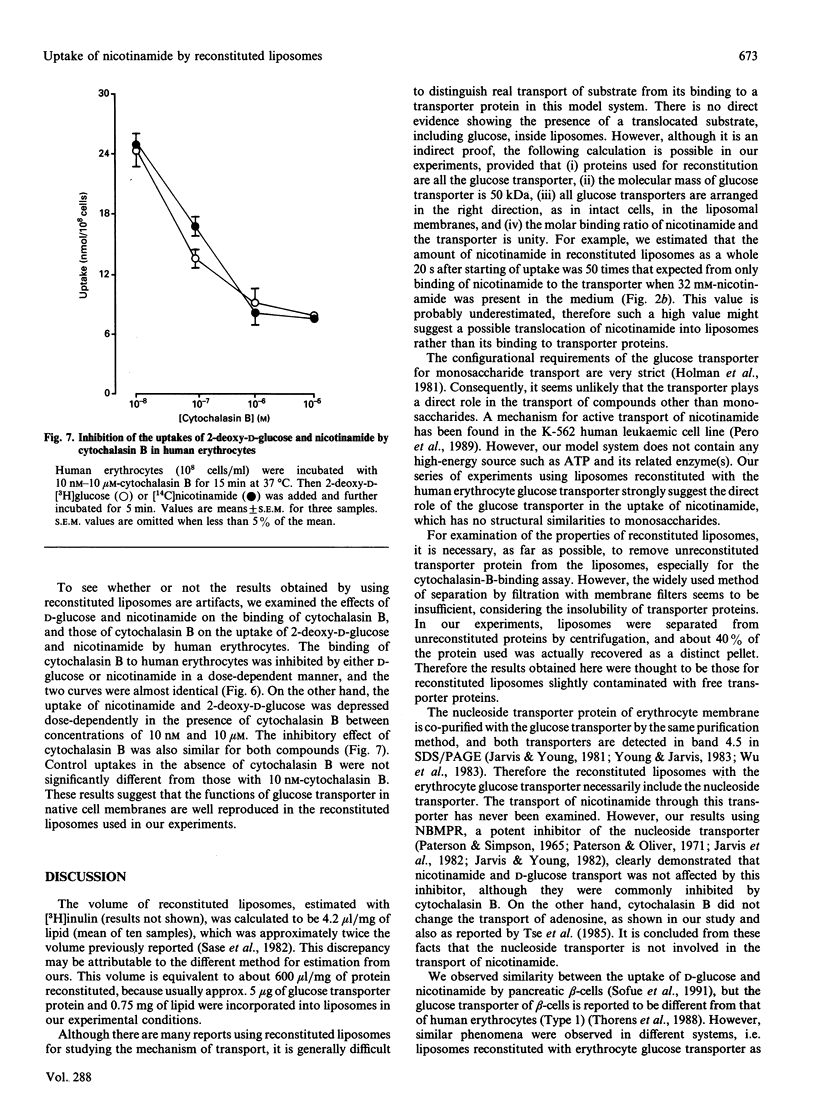
A kinetic study of the uptake of nicotinamide by reconstituted liposomes containing the human erythrocyte glucose transporter, compared with that of D-glucose, demonstrated that the Km and Vmax. values were almost the same for each compound, and that the uptake of D-glucose was competitively inhibited by nicotinamide. At 20 mM concentration, 2-deoxy-D-glucose, 3-O-methyl-D-glucose and 4,6-O-ethylidene-D-glucose all caused 50% inhibition of nicotinamide uptake, but L-glucose and nicotinic acid were not inhibitory. Similar results were obtained for the uptake of D-glucose. Cytochalasin B binding to the liposomes was inhibited in a dose-dependent manner by either nicotinamide or D-glucose. Antibody for glucose transporter detected in band 4.5 by SDS/PAGE inhibited the uptake of D-glucose and nicotinamide. A possible uptake of nicotinamide by nucleoside transporter was excluded. In human erythrocytes, cytochalasin B binding was inhibited dose-dependently by either nicotinamide or D-glucose, and cytochalasin B depressed the uptake of both nicotinamide and 2-deoxy-D-glucose. These findings were well reproduced in the reconstituted liposomes. The very close similarities between uptake of nicotinamide and D-glucose suggest that the glucose transporter plays a direct role in transport of nicotinamide, which is structurally quite different from monosaccharides, and thus that the transporter is probably multifunctional.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin J. M., Lienhard G. E., Baldwin S. A. The monosaccharide transport system of the human erythrocyte. Orientation upon reconstitution. Biochim Biophys Acta. 1980 Jul;599(2):699–714. doi: 10.1016/0005-2736(80)90211-4. [DOI] [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick A. P., Harik S. I., Klip A., Walker D. M. Identification and characterization of the glucose transporter of the blood-brain barrier by cytochalasin B binding and immunological reactivity. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7233–7237. doi: 10.1073/pnas.81.22.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunfeld C., Shigenaga J. K. Nicotinamide and other inhibitors of ADP-ribosylation block deoxyglucose uptake in cultured cells. Biochem Biophys Res Commun. 1984 Sep 17;123(2):785–791. doi: 10.1016/0006-291x(84)90298-5. [DOI] [PubMed] [Google Scholar]
- Holman G. D., Pierce E. J., Rees W. D. Spatial requirements for insulin-sensitive sugar transport in rat adipocytes. Biochim Biophys Acta. 1981 Sep 7;646(3):382–388. doi: 10.1016/0005-2736(81)90306-0. [DOI] [PubMed] [Google Scholar]
- Holman G. D., Rees W. D. Side-specific analogues for the rat adipocyte sugar transport system. Biochim Biophys Acta. 1982 Feb 8;685(1):78–86. doi: 10.1016/0005-2736(82)90037-2. [DOI] [PubMed] [Google Scholar]
- Jarvis S. M., McBride D., Young J. D. Erythrocyte nucleoside transport: asymmetrical binding of nitrobenzylthioinosine to nucleoside permeation sites. J Physiol. 1982 Mar;324:31–46. doi: 10.1113/jphysiol.1982.sp014099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis S. M., Young J. D. Extraction and partial purification of the nucleoside-transport system from human erythrocytes based on the assay of nitrobenzylthioinosine-binding activity. Biochem J. 1981 Jan 15;194(1):331–339. doi: 10.1042/bj1940331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis S. M., Young J. D. Nucleoside translocation in sheep reticulocytes and fetal erythrocytes: a proposed model for the nucleoside transporter. J Physiol. 1982 Mar;324:47–66. doi: 10.1113/jphysiol.1982.sp014100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara M., Hinkle P. C. Reconstitution and purification of the D-glucose transporter from human erythrocytes. J Biol Chem. 1977 Oct 25;252(20):7384–7390. [PubMed] [Google Scholar]
- Kawada J., Okita M., Nishida M., Yoshimura Y., Toyooka K., Kubota S. Protective effect of 4,6-O-ethylidene glucose against the cytotoxicity of streptozotocin in pancreatic beta cells in vivo: indirect evidence for the presence of a glucose transporter in beta cells. J Endocrinol. 1987 Mar;112(3):375–378. doi: 10.1677/joe.0.1120375. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Paterson A. R., Oliver J. M. Nucleoside transport. II. Inhibition by p-nitrobenzylthioguanosine and related compounds. Can J Biochem. 1971 Feb;49(2):271–274. doi: 10.1139/o71-039. [DOI] [PubMed] [Google Scholar]
- Paterson A. R., Simpson A. I. Inhibition of ribonucleoside metabolism in Ehrlich ascites tumor cells by purine analogue ribonucleosides. Can J Biochem. 1965 Oct;43(10):1701–1710. doi: 10.1139/o65-188. [DOI] [PubMed] [Google Scholar]
- Permutt M. A., Koranyi L., Keller K., Lacy P. E., Scharp D. W., Mueckler M. Cloning and functional expression of a human pancreatic islet glucose-transporter cDNA. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8688–8692. doi: 10.1073/pnas.86.22.8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sase S., Anraku Y., Nagano M., Osumi M., Kasahara M. Random distribution of the glucose transporter of human erythrocytes in reconstituted liposomes. J Biol Chem. 1982 Sep 25;257(18):11100–11105. [PubMed] [Google Scholar]
- Sofue M., Yoshimura Y., Nishida M., Kawada J. Uptake of nicotinamide by rat pancreatic beta cells with regard to streptozotocin action. J Endocrinol. 1991 Oct;131(1):135–138. doi: 10.1677/joe.0.1310135. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Kant J. A. Preparation of impermeable ghosts and inside-out vesicles from human erythrocyte membranes. Methods Enzymol. 1974;31:172–180. doi: 10.1016/0076-6879(74)31019-1. [DOI] [PubMed] [Google Scholar]
- Thorens B., Sarkar H. K., Kaback H. R., Lodish H. F. Cloning and functional expression in bacteria of a novel glucose transporter present in liver, intestine, kidney, and beta-pancreatic islet cells. Cell. 1988 Oct 21;55(2):281–290. doi: 10.1016/0092-8674(88)90051-7. [DOI] [PubMed] [Google Scholar]
- Tse C. M., Belt J. A., Jarvis S. M., Paterson A. R., Wu J. S., Young J. D. Reconstitution studies of the human erythrocyte nucleoside transporter. J Biol Chem. 1985 Mar 25;260(6):3506–3511. [PubMed] [Google Scholar]
- Wheeler T. J., Hinkle P. C. Kinetic properties of the reconstituted glucose transporter from human erythrocytes. J Biol Chem. 1981 Sep 10;256(17):8907–8914. [PubMed] [Google Scholar]
- Wu J. S., Jarvis S. M., Young J. D. The human erythrocyte nucleoside and glucose transporters are both band 4.5 membrane polypeptides. Biochem J. 1983 Sep 15;214(3):995–997. doi: 10.1042/bj2140995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. D., Jarvis S. M. Nucleoside transport in animal cells. Biosci Rep. 1983 Apr;3(4):309–322. doi: 10.1007/BF01122895. [DOI] [PubMed] [Google Scholar]


