Extended Data Fig. 3 |. Vector-copy-number quantification, immune-marker profiling, and functionality testing of MAJESTIC-produced CD8 CAR T cells.
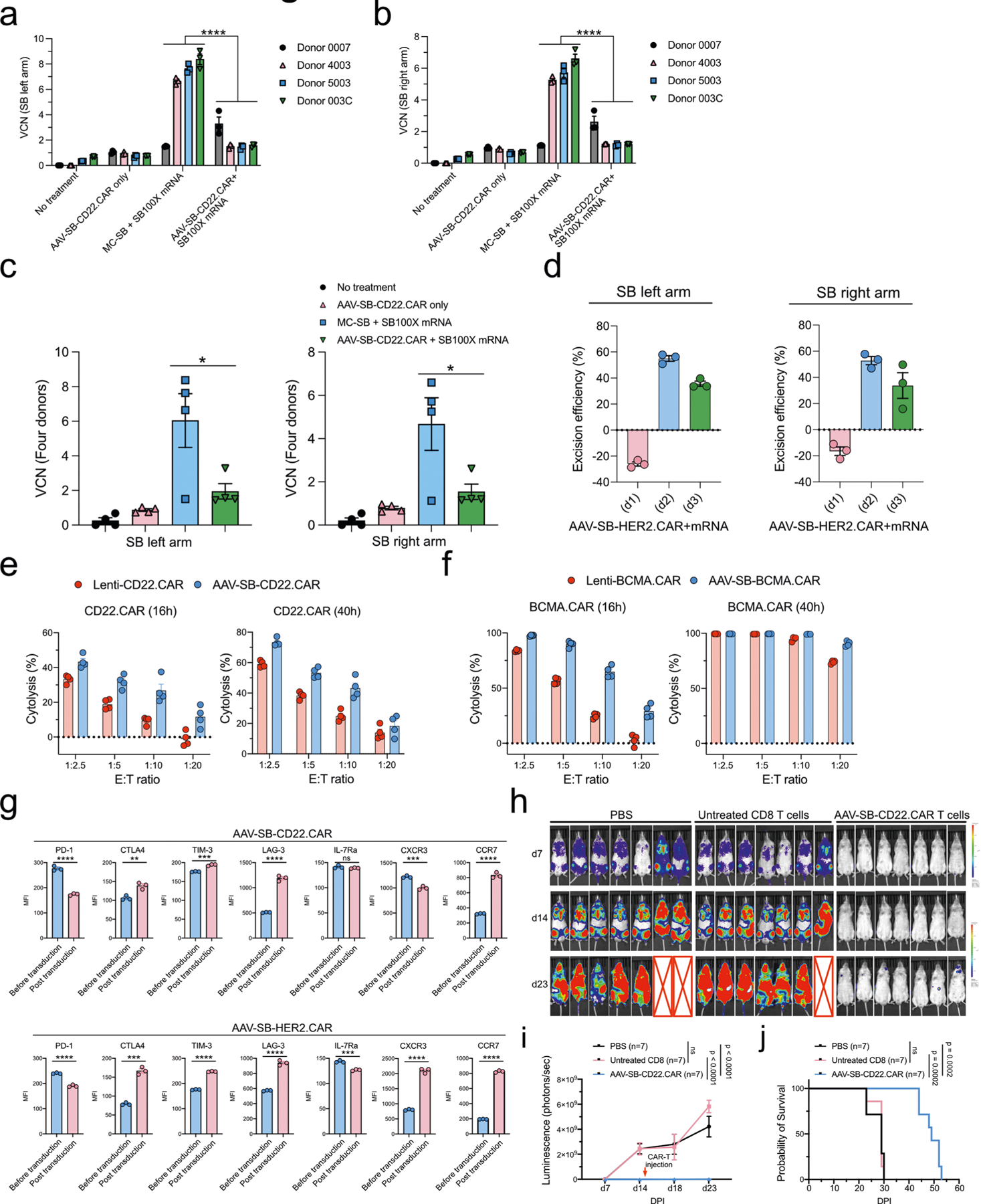
a-c, Vector copy number (VCN) quantification of MAJESTIC-manufactured CAR-T cells. Purified CAR T cells were collected for DNA extraction after three weeks of mRNA electroporation and viral transduction. (a) left arm probe, (b) right arm probe, (c) left panel: left arm probe, right panel: right arm probe. d, SB100X transposase excision efficiency evaluation. Left panel: left arm probe, right panel: right arm probe. e, Cytolysis analysis of NAML6-GL (NAML6 with GFP and luciferase reporters) cancer cells that were co-cultured with Lenti-CD22.CAR and AAV-SB-CD22.CAR T cells. CAR-Ts were seeded at various effector:target (E:T) ratios, and luciferase imaging was performed at two time points (16h and 40h). f, Cytolysis analysis of MM.1R-GL (MM.1R with GFP and luciferase reporters) cancer cells that were co-cultured with Lenti-BCMA.CAR and AAV-SB-BCMA.CAR T cells. CAR-Ts were seeded at various effector : target (E:T) ratios, and luciferase imaging was performed at two time points (16h and 40h). g, Exhaustion and memory marker expression in CD22-CAR and HER2-CAR T cells before and post transfection. Unpaired t tests were performed to evaluate statistical significance. h, Bioluminescent density of NSG mice that were injected with NALM6-GL cancer cells and with CD22-CAR therapy (n = 7 mice per group). i, Quantification of total luminescence for (h). n = 7 mice. Two-way ANOVA with multiple comparisons tests was performed to evaluate statistical significance. j, Survival curve of NALM6-GL-induced leukemia-bearing NSG mice that treated with PBS, untreated CD8 T cells, and AAV-SB-CD22.CAR T cells. Log-rank (Mantel-Cox) tests were performed to evaluate statistical significance. Donor 0007, 4003, 5003, 003C, 0286 T cells were used in this figure. Significance notes: ns - not significant; * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
