Abstract
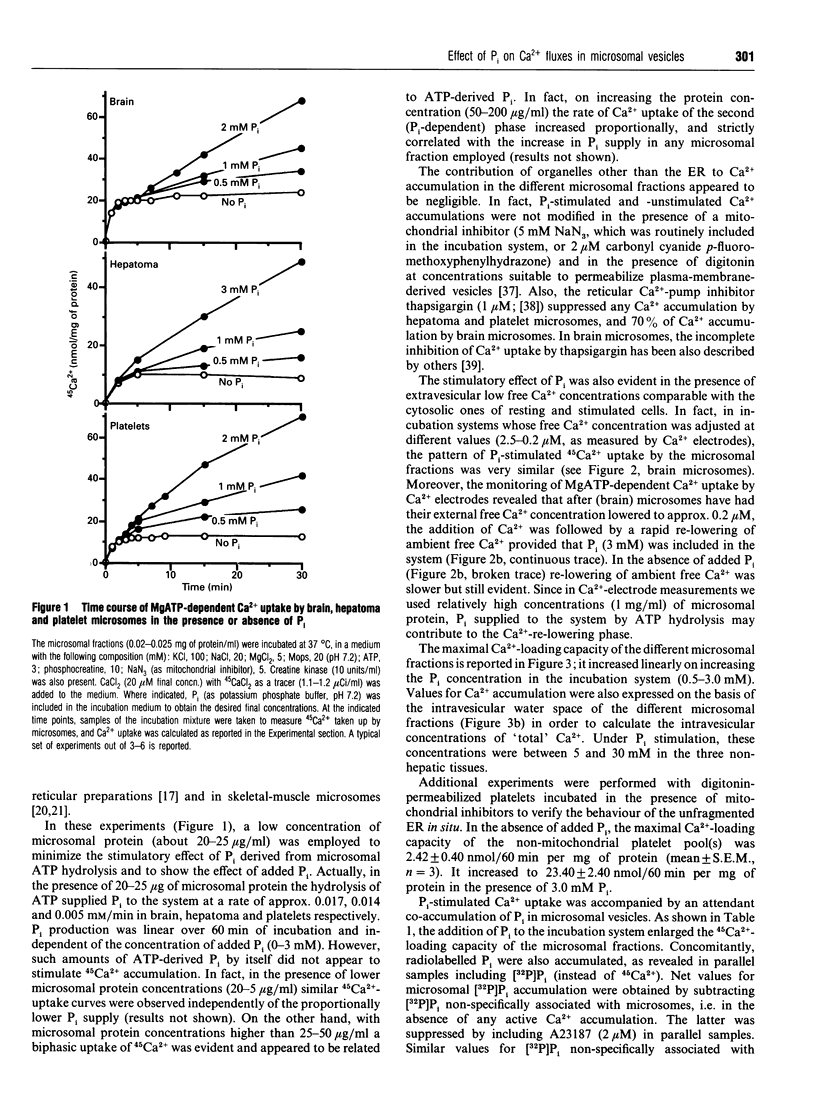
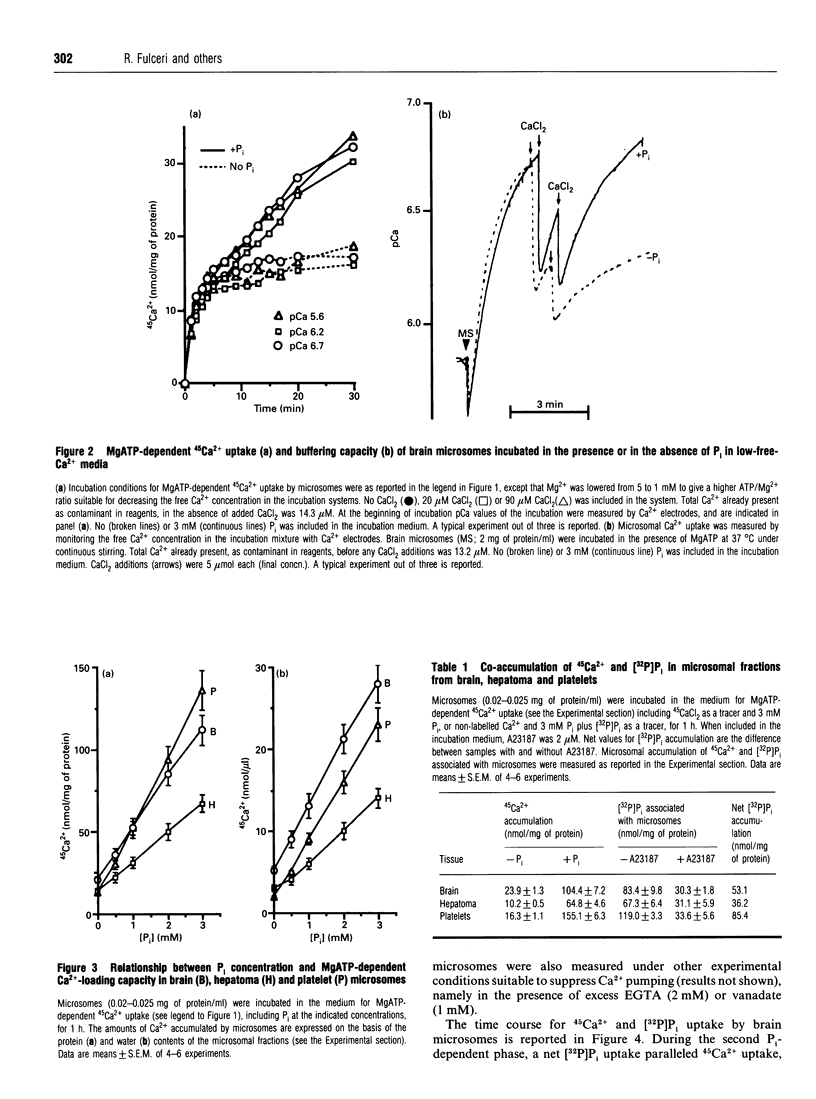
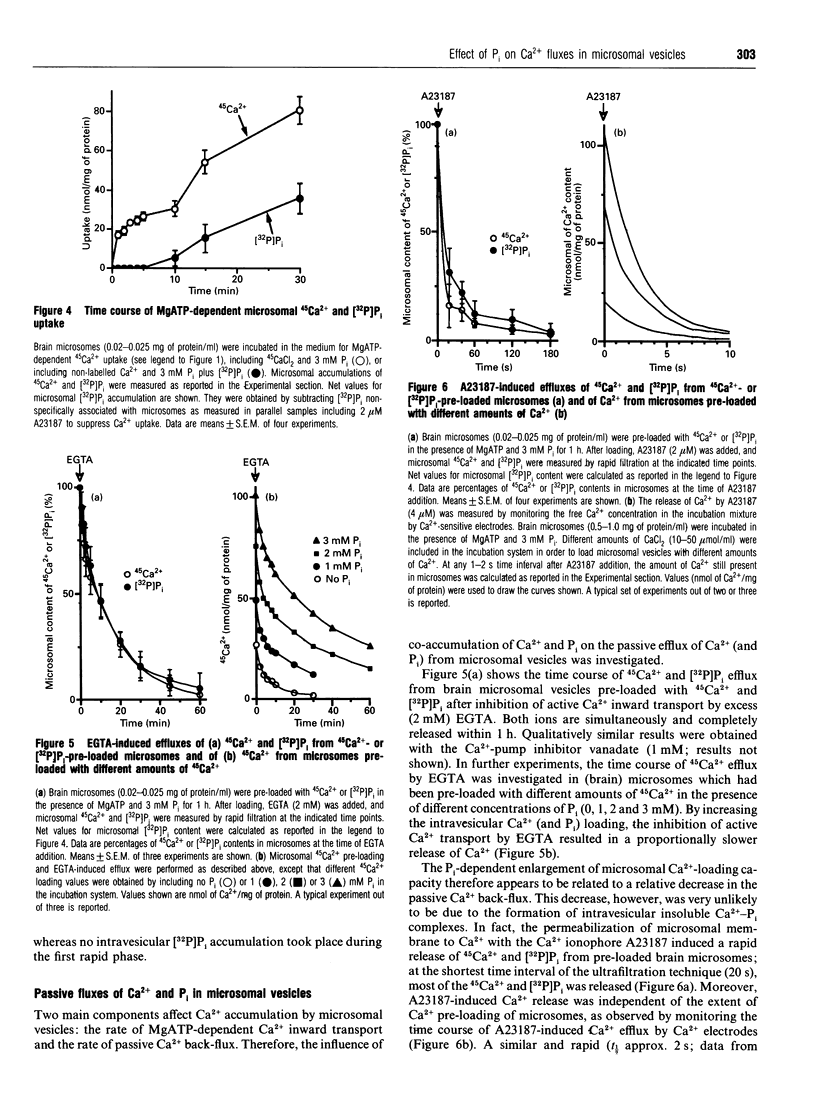
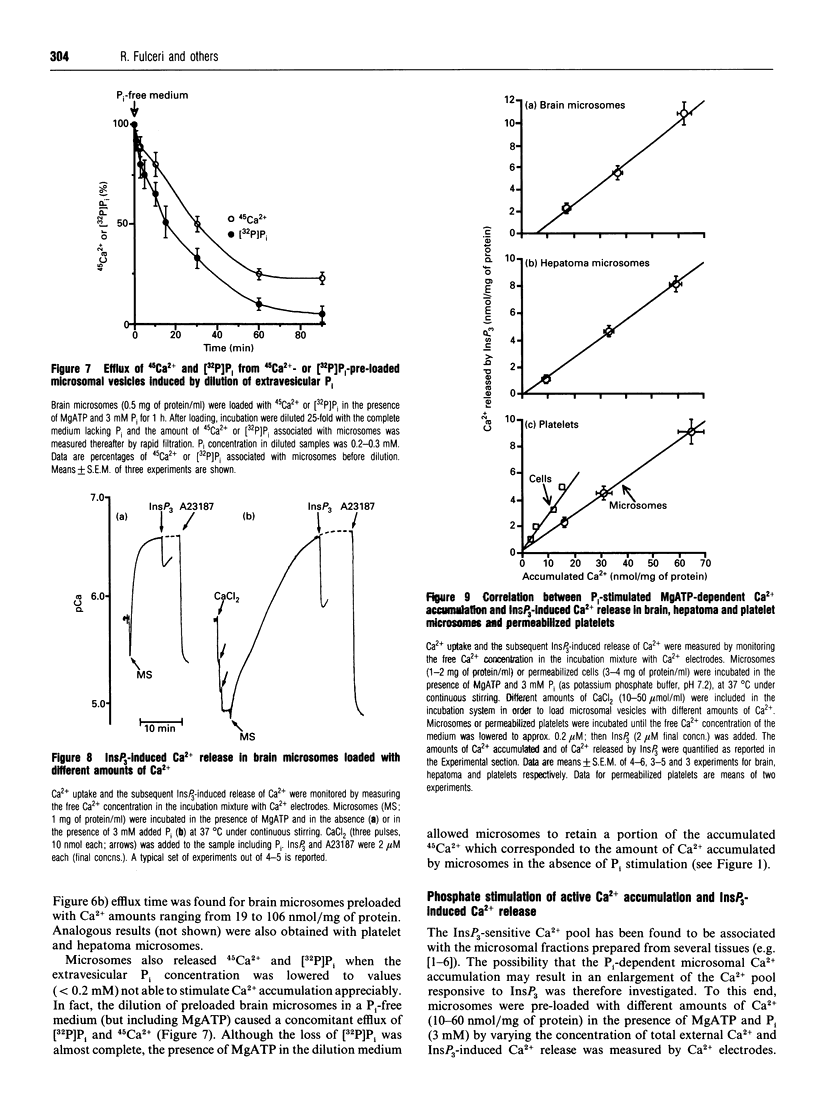
1. MgATP-dependent 45Ca2+ uptake by microsomes obtained from various non-hepatic tissues, namely rat brain, rat solid Morris hepatoma 3924A and human platelets, was measured in the presence of P(i) at low, cytosol-like, concentrations. 2. Increasing P(i) concentrations (0.5-3 mM) caused a progressive enlargement of the 45Ca(2+)-storage capacity of all the microsomal fractions. 3. As a result of P(i) stimulation of Ca2+ uptake, 45Ca2+ and [32P]P(i) were co-accumulated by the three microsomal fractions. 4. The time course for 45Ca2+ and [32P]P(i) accumulation in brain microsomes revealed a biphasic 45Ca2+ uptake: a rapid phase was followed by a second, slower, phase, which depended on the presence of P(i). During the P(i)-dependent phase, the uptake of 45Ca2+ was paralleled by the uptake of [32P]Pi. 5. The passive efflux of Ca2+ was paralleled by the efflux of P(i) and vice versa. In fact, the inhibition of active Ca2+ uptake by excess EGTA, or lowering the P(i) concentration of the incubation system by dilution, caused the release of 45Ca2+ and [32P]P(i) from 45Ca2+ or [32P]P(i) pre-loaded brain microsomes. The Ca2+ ionophore A23187 also released 45Ca2+ and [32P]P(i). 6. Ca2+ efflux by A23187 was rapid (t 1/2 approx. 2 s) and independent of the extent of intravesicular Ca2+ loading, which indicates that Ca2+ and P(i) do not form intravesicular insoluble complexes. 7. The progressive increase in Ca2+ accumulation, depending on P(i) stimulation, resulted in a proportional increase in the amount of Ca2+ releasable by InsP3 in the three non-hepatic microsomal fractions and in digitonin-permeabilized platelets. 8. Concomitantly to Ca2+, microsomal P(i) was also released by InsP3.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerboom T. P., Bookelman H., Zuurendonk P. F., van der Meer R., Tager J. M. Intramitochondrial and extramitochondrial concentrations of adenine nucleotides and inorganic phosphate in isolated hepatocytes from fasted rats. Eur J Biochem. 1978 Mar 15;84(2):413–420. doi: 10.1111/j.1432-1033.1978.tb12182.x. [DOI] [PubMed] [Google Scholar]
- Baumann O., Walz B., Somlyo A. V., Somlyo A. P. Electron probe microanalysis of calcium release and magnesium uptake by endoplasmic reticulum in bee photoreceptors. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):741–744. doi: 10.1073/pnas.88.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti A., Fulceri R., Comporti M. Calcium sequestration activity in rat liver microsomes. Evidence for a cooperation of calcium transport with glucose-6-phosphatase. Biochim Biophys Acta. 1985 Jun 27;816(2):267–277. doi: 10.1016/0005-2736(85)90494-8. [DOI] [PubMed] [Google Scholar]
- Benedetti A., Fulceri R., Romani A., Comporti M. MgATP-dependent glucose 6-phosphate-stimulated Ca2+ accumulation in liver microsomal fractions. Effects of inositol 1,4,5-trisphosphate and GTP. J Biol Chem. 1988 Mar 5;263(7):3466–3473. [PubMed] [Google Scholar]
- Berridge M. J. Inositol lipids and cell proliferation. Biochim Biophys Acta. 1987 Apr 20;907(1):33–45. doi: 10.1016/0304-419x(87)90017-5. [DOI] [PubMed] [Google Scholar]
- Brattin W. J., Jr, Waller R. L., Recknagel R. O. Analysis of microsomal calcium sequestration by steady state isotope exchange. Enzyme kinetics and role of membrane permeability. J Biol Chem. 1982 Sep 10;257(17):10044–10051. [PubMed] [Google Scholar]
- Brattin W. J., Waller R. L. Calcium inhibition of rat liver microsomal calcium-dependent ATPase. J Biol Chem. 1983 Jun 10;258(11):6724–6729. [PubMed] [Google Scholar]
- Carafoli E. Intracellular calcium homeostasis. Annu Rev Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- Cerdan S., Seelig J. NMR studies of metabolism. Annu Rev Biophys Biophys Chem. 1990;19:43–67. doi: 10.1146/annurev.bb.19.060190.000355. [DOI] [PubMed] [Google Scholar]
- Chance B., Nakase Y., Bond M., Leigh J. S., Jr, McDonald G. Detection of 31P nuclear magnetic resonance signals in brain by in vivo and freeze-trapped assays. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4925–4929. doi: 10.1073/pnas.75.10.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C. C., Malloy C. R., Radda G. K. Effect of fasting and acute ethanol administration on the energy state of in vivo liver as measured by 31P-NMR spectroscopy. Biochim Biophys Acta. 1986 Jan 23;885(1):12–22. doi: 10.1016/0167-4889(86)90033-9. [DOI] [PubMed] [Google Scholar]
- Dean W. L. Purification and reconstitution of a Ca2+ pump from human platelets. J Biol Chem. 1984 Jun 10;259(11):7343–7348. [PubMed] [Google Scholar]
- Dienel G. A., Nelson T., Cruz N. F., Jay T., Crane A. M., Sokoloff L. Over-estimation of glucose-6-phosphatase activity in brain in vivo. Apparent difference in rates of [2-3H]glucose and [U-14C]glucose utilization is due to contamination of precursor pool with 14C-labeled products and incomplete recovery of 14C-labeled metabolites. J Biol Chem. 1988 Dec 25;263(36):19697–19708. [PubMed] [Google Scholar]
- Enouf J., Lebret M., Bredoux R., Levy-Toledano S., Caen J. P. Abnormal calcium transport into microsomes of grey platelet syndrome. Br J Haematol. 1987 Apr;65(4):437–440. doi: 10.1111/j.1365-2141.1987.tb04146.x. [DOI] [PubMed] [Google Scholar]
- Feher J. J., Lipford G. B. Calcium oxalate and calcium phosphate capacities of cardiac sarcoplasmic reticulum. Biochim Biophys Acta. 1985 Sep 10;818(3):373–385. doi: 10.1016/0005-2736(85)90012-4. [DOI] [PubMed] [Google Scholar]
- Fulceri R., Bellomo G., Gamberucci A., Benedetti A. MgATP-dependent accumulation of calcium ions and inorganic phosphate in a liver reticular pool. Biochem J. 1990 Dec 1;272(2):549–552. doi: 10.1042/bj2720549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulceri R., Romani A., Pompella A., Benedetti A. Glucose 6-phosphate stimulation of MgATP-dependent Ca2+ uptake by rat kidney microsomes. Biochim Biophys Acta. 1990 Feb 16;1022(1):129–133. doi: 10.1016/0005-2736(90)90409-h. [DOI] [PubMed] [Google Scholar]
- Inamitsu T., Ohtsuki I. Characterization of ATP-dependent Ca2+ uptake by canine brain microsomes with saponin. Eur J Biochem. 1984 Nov 15;145(1):115–121. doi: 10.1111/j.1432-1033.1984.tb08529.x. [DOI] [PubMed] [Google Scholar]
- Inesi G., de Meis L. Regulation of steady state filling in sarcoplasmic reticulum. Roles of back-inhibition, leakage, and slippage of the calcium pump. J Biol Chem. 1989 Apr 5;264(10):5929–5936. [PubMed] [Google Scholar]
- Joseph S. K., Williamson J. R. Inositol polyphosphates and intracellular calcium release. Arch Biochem Biophys. 1989 Aug 15;273(1):1–15. doi: 10.1016/0003-9861(89)90156-2. [DOI] [PubMed] [Google Scholar]
- Kijima Y., Ogunbunmi E., Fleischer S. Drug action of thapsigargin on the Ca2+ pump protein of sarcoplasmic reticulum. J Biol Chem. 1991 Dec 5;266(34):22912–22918. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lanzetta P. A., Alvarez L. J., Reinach P. S., Candia O. A. An improved assay for nanomole amounts of inorganic phosphate. Anal Biochem. 1979 Nov 15;100(1):95–97. doi: 10.1016/0003-2697(79)90115-5. [DOI] [PubMed] [Google Scholar]
- Lawry T. J., Karczmar G. S., Weiner M. W., Matson G. B. Computer simulation of MRS localization techniques: an analysis of ISIS. Magn Reson Med. 1989 Mar;9(3):299–314. doi: 10.1002/mrm.1910090302. [DOI] [PubMed] [Google Scholar]
- Meldolesi J., Madeddu L., Pozzan T. Intracellular Ca2+ storage organelles in non-muscle cells: heterogeneity and functional assignment. Biochim Biophys Acta. 1990 Nov 12;1055(2):130–140. doi: 10.1016/0167-4889(90)90113-r. [DOI] [PubMed] [Google Scholar]
- Mermier P., Hasselbach W. The biphasic active transport of calcium by the fragmented sarcoplasmic reticulum as revealed by the flow dialysis method. Eur J Biochem. 1976 May 1;64(2):613–620. doi: 10.1111/j.1432-1033.1976.tb10341.x. [DOI] [PubMed] [Google Scholar]
- Mirabelli F., Salis A., Vairetti M., Bellomo G., Thor H., Orrenius S. Cytoskeletal alterations in human platelets exposed to oxidative stress are mediated by oxidative and Ca2+-dependent mechanisms. Arch Biochem Biophys. 1989 May 1;270(2):478–488. doi: 10.1016/0003-9861(89)90529-8. [DOI] [PubMed] [Google Scholar]
- Muallem S., Schoeffield M., Pandol S., Sachs G. Inositol trisphosphate modification of ion transport in rough endoplasmic reticulum. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4433–4437. doi: 10.1073/pnas.82.13.4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordlie R. C. Multifunctional glucose-6-phosphatase: cellular biology. Life Sci. 1979 Jun 25;24(26):2397–2404. doi: 10.1016/0024-3205(79)90447-8. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Capacitative calcium entry revisited. Cell Calcium. 1990 Nov-Dec;11(10):611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- Shah J., Cohen R. S., Pant H. C. Inositol trisphosphate-induced calcium release in brain microsomes. Brain Res. 1987 Sep 1;419(1-2):1–6. doi: 10.1016/0006-8993(87)90562-2. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Bond M., Somlyo A. V. Calcium content of mitochondria and endoplasmic reticulum in liver frozen rapidly in vivo. Nature. 1985 Apr 18;314(6012):622–625. doi: 10.1038/314622a0. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V., Bond M., Broderick R., Goldman Y. E., Shuman H., Walker J. W., Trentham D. R. Calcium and magnesium movements in cells and the role of inositol trisphosphate in muscle. Soc Gen Physiol Ser. 1987;42:77–92. [PubMed] [Google Scholar]
- Trotta E. E., de Meis L. ATP-dependent calcium accumulation in brain microsomes. Enhancement by phosphate and oxalate. Biochim Biophys Acta. 1975 Jun 25;394(2):239–247. doi: 10.1016/0005-2736(75)90262-x. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Rink T. J. Ca2+-selective electrodes: a novel PVC-gelled neutral carrier mixture compared with other currently available sensors. J Neurosci Methods. 1981 Jun;4(1):73–86. doi: 10.1016/0165-0270(81)90020-0. [DOI] [PubMed] [Google Scholar]
- Veech R. L., Lawson J. W., Cornell N. W., Krebs H. A. Cytosolic phosphorylation potential. J Biol Chem. 1979 Jul 25;254(14):6538–6547. [PubMed] [Google Scholar]
- Wolf B. A., Colca J. R., Comens P. G., Turk J., McDaniel M. L. Glucose 6-phosphate regulates Ca2+ steady state in endoplasmic reticulum of islets. A possible link in glucose-induced insulin secretion. J Biol Chem. 1986 Dec 15;261(35):16284–16287. [PubMed] [Google Scholar]
- de Meis L., Hasselbach W., Machado R. D. Characterization of calcium oxalate and calcium phosphate deposits in sarcoplasmic reticulum vesicles. J Cell Biol. 1974 Aug;62(2):505–509. doi: 10.1083/jcb.62.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]


