Abstract
The impact of age (≥ 85 vs < 85 years) on clinical outcomes and pacemaker performance of conduction system pacing (CSP) compared to right ventricular pacing (RVP) were examined. Consecutive patients from a prospective, observational, multicenter study with pacemakers implanted for bradycardia were studied. The primary endpoint was a composite of heart failure (HF)-hospitalizations, pacing-induced cardiomyopathy requiring cardiac resynchronization therapy or all-cause mortality. Secondary endpoints were acutely successful CSP, absence of pacing-complications, optimal pacemaker performance defined as pacing thresholds < 2.5 V, R-wave amplitude ≥ 5 V and absence of complications, threshold stability (no increases of > 1 V) and persistence of His-Purkinje capture on follow-up. Among 984 patients (age 74.1 ± 11.2 years, 41% CSP, 16% ≥ 85 years), CSP was independently associated with reduced hazard of the primary endpoint compared to RVP, regardless of age-group (< 85 years: adjusted hazard ratio [AHR] 0.63, 95% confidence interval [CI] 0.40–0.98; ≥ 85 years: AHR 0.40, 95% CI 0.17–0.94). Among patients with CSP, age did not significantly impact the secondary endpoints of acute CSP success (86% vs 88%), pacing complications (19% vs 11%), optimal pacemaker performance (64% vs 69%), threshold stability (96% vs 96%) and persistent His-Purkinje capture (86% vs 91%) on follow-up (all p > 0.05). CSP improves clinical outcomes in all age-groups, without compromising procedural safety or pacemaker performance in the very elderly.
Subject terms: Cardiology, Cardiac device therapy
Introduction
With improved medical care and increased life expectancy, the prevalence of age-related arrhythmias in the very elderly, including conduction system disorders, is expected to rise exponentially1,2. In the United States, the number of very elderly individuals ≥ 85 years is expected to triple to 19 million people by 20603. This presents a significant challenge to healthcare systems, as very elderly patients make up > 40% of pacemaker implants4.
Early population-based studies more than a decade ago on pacemaker outcomes were focused primarily on mortality in this vulnerable group of patients5–11. Attempts to improve survival in the elderly with “physiologic”, dual chamber pacing, compared to single chamber pacing, were unsuccessful in yielding significant therapeutic differences7. However, little is known about pacing-associated morbidity in the very elderly, including heart failure (HF) and pacing-induced cardiomyopathy (PCM). This is particularly pertinent in healthcare delivery for the ageing global population, particularly in advanced countries.
Conduction system pacing (CSP), which provides greater physiological ventricular activation, has been associated with reduced HF and improved survival among patients with bradycardia12–14. CSP may be an attractive option in this group of patients, but its feasibility, safety and efficacy in the very elderly has not been systemically studied. A more contemporaneous study of age-related effects in pacing outcomes and in regard to newer pacing modalities is required.
The aims of this study were therefore, to determine (i) if the clinical benefits of CSP compared to RVP applied similarly to very elderly patients (≥ 85 years) as younger patients (< 85 years), and (ii), to examine age-related differences in optimal pacemaker performance and safety profile between CSP and RVP, in patients with bradycardia and preserved left ventricular ejection fraction (LVEF) of ≥ 50%.
Methods
Study population
This was a multicenter, observational, prospective registry of cardiac implantable devices involving 3 major public hospitals providing healthcare to Eastern and Western Singapore. Consecutive patients who underwent RVP or CSP from 2016 to 2022 were included in this study and the inclusion of allcomers presenting to public hospitals minimized selection bias. From 2016, RVP was the conventional pacing modality, with His-bundle pacing (HBP) adopted from 2018 and left bundle branch pacing (LBBP) from 202012,15,16. The choice of pacing modality (CSP or RVP) was based on implanting physician discretion. Only participants with de novo pacemakers indicated for sinus node dysfunction (SND) or atrioventricular block (AVB) with preserved LVEF (≥ 50%) were included. The study was approved by the local institutional review board (National Healthcare Group Domain Specific Review Board: 2020/00211) and in compliance with the Declaration of Helsinki. All patients provided informed consent.
Pacemaker implantation
RVP and CSP were performed as previously described12,15. With RVP, the RV lead was positioned at either the RV apex or outflow tract. CSP was performed with either fixed (SelectSecure 3830 leads via C315 His sheath [Medtronic Inc.]) or extendable helix leads (Solia S lead via Selectra 3D sheath [Biotronik SE & Co.] or Tendril STS lead via Agilis HisPro catheter [St. Jude Medical/Abbott]). Prior to deployment, extendable helix leads were prepared in accordance with manufacturer guidelines17. For both HBP and LBBP, the same clockwise rotational techniques were used to achieve septal penetration. Acute HBP success was defined as selective or non-selective HB capture at pacing thresholds of < 2.5 V at 1 ms. Acute LBBP success was defined by presence of “rsR” pattern in lead V1, with either LB potential, transitions in paced QRS morphology with decreasing pacing outputs or programmed ventricular electrical stimulation indicating selective/non-selective LBB capture to myocardial capture, abrupt shortening of LV activation time to < 75 ms or V6-V1 interpeak interval of > 33 ms18. Patients who did not meet acute HBP/LBBP success criteria were classified as RVP.
Pacing characteristics
Pacing thresholds, R-wave amplitudes and lead impedance were recorded at baseline and on follow-up. Optimal pacemaker performance was defined as having fulfilled all of R-wave amplitude ≥ 5 V to avoid undersensing, pacing threshold < 2.5 V for preservation of battery longevity, and absence of any pacemaker-related complications15. In patients who were pacing dependent without a recordable R-wave, satisfaction of the latter 2 criteria was sufficient to fulfil optimal pacemaker performance. Periprocedural and late complications were recorded. Threshold stability was defined as absence of increased pacing thresholds by > 1 V on follow-up. Specific to CSP, enduring conduction system capture was determined during pacemaker clinic visits19. Loss of conduction system capture was considered a complication in defining optimal pacemaker performance.
Clinical endpoints
The primary endpoint of this study was a composite of HF-hospitalizations, PCM (absolute decline in LVEF by ≥ 10% from baseline to LVEF < 50%) requiring upgrade to CRT or all-cause mortality. Secondary endpoints were acute CSP success, pacing-complications, optimal pacemaker performance, pacing threshold stability and enduring conduction system capture on follow-up. All clinical endpoints were ascertained through linked hospital electronic records and discharge summaries. Follow-up was censored at time of first clinical event (PCM, HF-hospitalization or death) or last clinical contact in those without a primary endpoint event.
Statistical analysis
Baseline characteristics across the age-groups were compared with the Wilcoxon rank-sum test (continuous) or chi-squared test (categorical). Clinical endpoints were evaluated in per-treatment analyses. Time to primary endpoint and its individual components of HF-hospitalization and all-cause mortality were evaluated in Cox proportional hazard models. Forest plots and Kaplan–Meier survival curves were plotted for the predictors of designated clinical endpoints. Comparison of the incidence of secondary endpoints were performed by chi-squared tests. Logistic regression models were constructed to evaluate the association of age with optimal device performance. Statistical analyses were performed with STATA MP v16 (StataCorp) at 5% level of significance.
Results
Of 984 patients with de novo pacemakers and LVEF ≥ 50% (mean age 74.1 ± 11.2 years, 49% female, 41% CSP, 59% RVP), 824 (84%) were < 85 years and 160 (16%) were ≥ 85 years. Baseline clinical characteristics were similar between those with received CSP and RVP in both age groups, except for a higher prevalence of hypertension among younger (< 85 years) patients who received RVP compared to CSP (p = 0.01), and a higher prevalence of AVB in those who received CSP compared to RVP in both age groups (p < 0.05, Table 1). LVEF was higher among those < 85 years with RVP compared to CSP but clinically insignificant (61.5 ± 5.3% vs 60.5 ± 4.5%, p = 0.03).
Table 1.
Comparison of baseline characteristics by age-groups.
| Total | < 85 years | ≥ 85 years | |||||
|---|---|---|---|---|---|---|---|
| CSP | RVP | p value | CSP | RVP | p value | ||
| N (%) | 984 | 325 | 499 | 76 | 84 | ||
| Age, years | 74.1 ± 11.2 | 71.4 ± 9.7 | 71.4 ± 10.4 | 0.617 | 88.2 ± 3.2 | 88.1 ± 2.7 | 0.836 |
| Female (%) | 482 (49) | 162 (50) | 230 (46) | 0.292 | 40 (53) | 50 (60) | 0.380 |
| Hypertension (%) | 762 (77) | 256 (79) | 372 (75) | 0.164 | 62 (82) | 72 (86) | 0.479 |
| Diabetes (%) | 371 (38) | 136 (42) | 182 (36) | 0.122 | 24 (32) | 29 (35) | 0.693 |
| AF (%) | 359 (36) | 116 (36) | 183 (37) | 0.775 | 25 (33) | 35 (42) | 0.252 |
| IHD (%) | 299 (30) | 79 (24) | 163 (33) | 0.010 | 23 (30) | 34 (40) | 0.178 |
| Prior HF (%) | 77 (8) | 23 (7) | 37 (7) | 0.855 | 7 (9) | 10 (12) | 0.581 |
| Stroke (%) | 154 (16) | 47 (15) | 77 (16) | 0.880 | 18 (25) | 12 (14) | 0.099 |
| COPD (%) | 35 (4) | 7 (2) | 20 (4) | 0.169 | 4 (5) | 5 (5) | 0.838 |
| Creatinine, μmol | 114.9 ± 119.3 | 113.6 ± 136.9 | 118.9 ± 122.1 | 0.518 | 98.9 ± 39.7 | 110.7 ± 64.3 | 0.343 |
| LBBB (%) | 38 (4) | 14 (4) | 17 (3) | 0.507 | 2 (3) | 5 (6) | 0.305 |
| LVEF, % | 61.2 ± 5.0 | 60.5 ± 4.5 | 61.5 ± 5.3 | 0.003 | 61.7 ± 4.9 | 61.9 ± 5.1 | 0.628 |
| LVESVI, ml/m2 | 21.6 ± 11.1 | 23.5 ± 14.0 | 20.9 ± 9.0 | 0.217 | 20.6 ± 10.3 | 19.6 ± 8.7 | 0.768 |
| Beta blockers (%) | 360 (37) | 114 (35) | 182 (36) | 0.707 | 27 (36) | 37 (44) | 0.272 |
| ACE-I/ARB/ARNI (%) | 458 (47) | 161 (50) | 234 (47) | 0.433 | 25 (33) | 38 (45) | 0.111 |
| Pacing indication (%) | < 0.001 | 0.005 | |||||
| SND | 489 (50) | 139 (43) | 279 (56) | 25 (33) | 46 (55) | ||
| AVB | 495 (50) | 186 (57) | 220 (44) | 51 (67) | 38 (45) | ||
| Device type (%) | 0.001 | 0.026 | |||||
| Single chamber (VVI mode) | 90 (9) | 14 (4) | 53 (11) | 6 (8) | 17 (20) | ||
| Dual chamber (DDD mode) | 894 (91) | 311 (96) | 446 (89) | 70 (92) | 67 (80) | ||
Significant values are in [bold].
Comparison baseline of characteristics expressed as mean ± standard deviation or percentages.
AF, Atrial fibrillation; ACE-I, Angiotensin-converting enzyme inhibitor; ARB, Angiotensin-II receptor blockers; ARNI, Angiotensin receptor-neprilysin inhibitor; AVB, Atrioventricular block; COPD, Chronic obstructive pulmonary disease; CSP, Conduction system pacing; HF, Heart failure; IHD, Ischemic heart disease; LBBB, Left bundle branch block; LVEF, LV ejection fraction; LVESVI, LV end-systolic volume index; RVP, Right ventricular pacing; SND, Sinus node dysfunction.
Primary endpoint
Over a mean follow-up of 955 ± 614 days (CSP: 554 ± 361 days; RVP: 1232 ± 600 days), the primary endpoint occurred in 192 (20%), HF-hospitalization in 98 (10%) and all-cause mortality in 118 (12%) patients. Among 335 patients with available repeat TTE on follow-up, 54 (16%) had PCM, with similar incidences between groups (p = 0.927). Of the 118 deaths, 46 (39%) were due to non-cardiovascular causes (37% in < 85 years vs 44% in ≥ 85 years), 26 (22%) due to cardiovascular causes (23% in < 85 years vs 19% in ≥ 85 years), while 46 (39%) of deaths were due to unknown causes (40% in < 85 years vs 38% in ≥ 85 years). Univariable associations of clinical covariates with the primary endpoint are presented in Supplementary Table 1, and CSP versus RVP with all clinical endpoints in Supplementary Table 2.
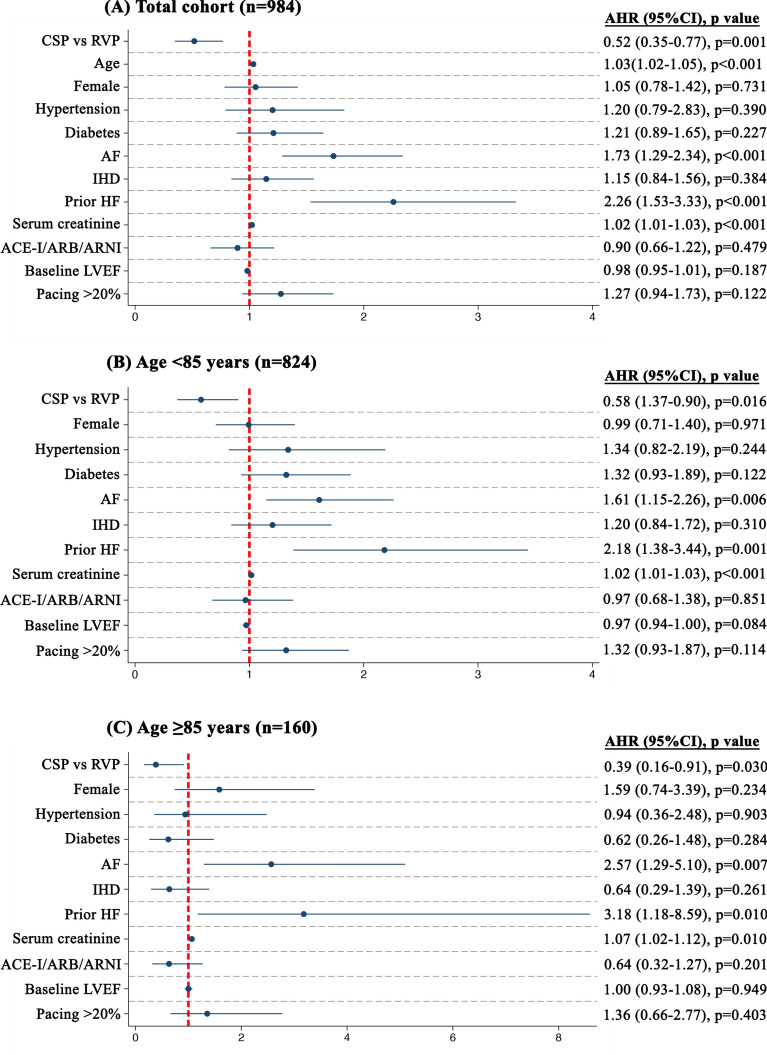
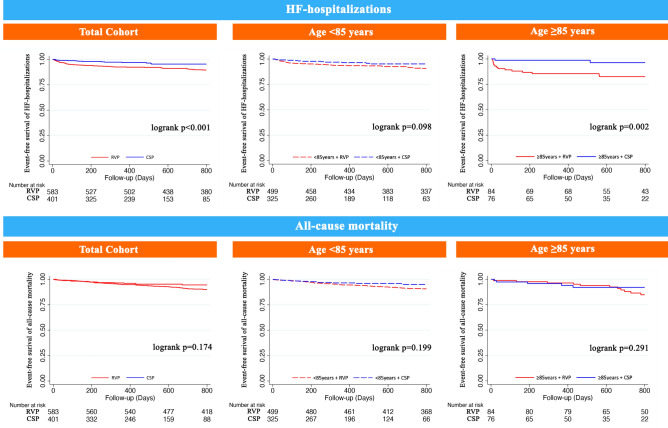
Compared to RVP, CSP was independently associated with reduced 48% reduced hazard of the primary endpoint (Fig. 1), 58% reduced hazard of HF-hospitalizations and trended towards lower all-cause mortality in the whole cohort (Table 2). There were no interactions with age with respect to each of the clinical endpoints (pinteraction for primary endpoint = 0.117, pinteraction for HF-hospitalizations = 0.059, pinteraction for all-cause mortality = 0.357). Stratified by age-group, CSP was independently associated with lower hazards of both the primary endpoint (Fig. 1) and HF-hospitalizations (p < 0.05), and trended towards lower hazards of all-cause mortality in both age-groups (p > 0.05, Table 2). Kaplan–Meier survival curves of the primary endpoint and the individual components of HF-hospitalizations and all-cause mortality are demonstrated in the Figs. 2 and 3, respectively.
Figure 1.
Association of age and pacing-modality with primary outcome. CSP was associated with reduced hazard of the primary outcome, without age-group differences by ≥ 85 years and < 85 years.
Table 2.
Association of age-groups with primary endpoint, HF-hospitalizations and all-cause-mortality.
| Primary outcome | HF-hospitalizations | All-cause mortality | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | AHR | 95% CI | p value | N (%) | AHR | 95% CI | p value | N (%) | AHR | 95% CI | p value | |
| Whole cohort* | ||||||||||||
| RVP | 157 (27) | Ref | Ref | Ref | 82 (14) | Ref | Ref | Ref | 97 (17) | Ref | Ref | Ref |
| CSP | 35 (9) | 0.52 | 0.35–0.77 | 0.001 | 16 (4) | 0.42 | 0.24–0.73 | 0.002 | 21 (5) | 0.64 | 0.38–1.08 | 0.096 |
| Age < 85 years† | ||||||||||||
| RVP | 120 (24) | Ref | Ref | Ref | 61 (12) | Ref | Ref | Ref | 71 (14) | Ref | Ref | Ref |
| CSP | 27 (8) | 0.58 | 0.37–0.90 | 0.016 | 14 (4) | 0.54 | 0.29–0.99 | 0.047 | 15 (5) | 0.62 | 0.34–1.15 | 0.132 |
| Age ≥ 85 years† | ||||||||||||
| RVP | 37 (44) | Ref | Ref | Ref | 21 (25) | Ref | Ref | Ref | 26 (31) | Ref | Ref | Ref |
| CSP | 8 (11) | 0.39 | 0.16–0.91 | 0.030 | 2 (3) | 0.15 | 0.03–0.64 | 0.011 | 6 (8) | 0.78 | 0.26–2.32 | 0.656 |
Significant values are in [bold].
Primary composite outcome of HF-hospitalizations, pacing-induced cardiomyopathy requiring upgrade to cardiac resynchronization therapy, or all-cause mortality.
*Multivariable Cox regression analysis adjusting for age, sex, hypertension, diabetes, AF, IHD, prior HF, serum creatinine per 10 μmol, ACE-I/ARB/ARNI, LVEF and pacing burden > 20%.
†Multivariable Cox regression analysis adjusting for sex, hypertension, diabetes, AF, IHD, prior HF, serum creatinine per 10 μmol, ACE-I/ARB/ARNI, LVEF and pacing burden > 20%.
AHR, adjusted hazard ratio; CI, confidence interval; Rest of abbreviations as per Table 1.
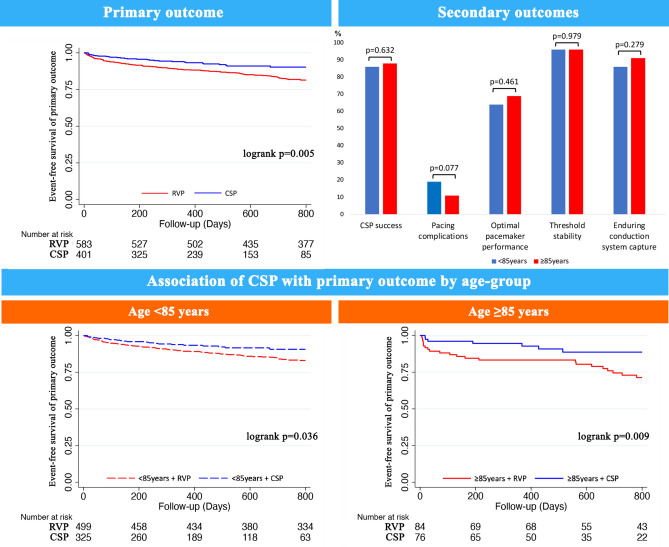
Figure 2.
Age-related pacing outcomes in the very elderly. CSP was independently associated with significantly lower hazard of the primary outcome of HF-hospitalizations, pacing-induced cardiomyopathy requiring CRT upgrade or all-cause mortality, compared to RVP. Increasing age (≥ 85 years vs < 85 years) did not significantly impact the beneficial effects of CSP or adversely compromise acute CSP success, pacing complications, optimal pacemaker function, threshold stability and enduring conduction system capture on follow-up.
Figure 3.
Associations of CSP compared to RVP with HF-hospitalizations and all-cause mortality. Kaplan–Meier survival curves of HF-hospitalizations and all-cause mortality stratified by age < 85 years and ≥ 85 years.
Pacing characteristics
Pacing parameters are shown in Table 3. Paced QRS duration was lower with CSP than RVP (108 ± 19 ms vs 125 ± 39 ms vs 108 ± 19 ms, p < 0.001), but did not differ by age-group (Table 3). There were no age-related differences in pacing threshold, R-wave amplitude at baseline and lead impedance on follow-up (p > 0.05), except for lower R-wave amplitudes (10.2 ± 5.2 mV vs 11.4 ± 5.4 mV, p = 0.025) on follow-up and lower lead impedance (686.5 ± 183.8 ohms vs 714.9 ± 184.6 ohms, p = 0.049) at baseline recorded in patients ≥ 85 years compared to < 85 years, respectively (Table 3). Ventricular pacing burden was significantly higher in the very elderly (p = 0.002), with the very elderly more frequently having a pacing burden of > 20% compared to younger patients (70% vs 53%, p < 0.001). Increased pacing thresholds by > 1 V on follow-up occurred in 32 (3%) patients (16 RVP, 15 HBP, 1 LBBP) and did not differ by age-groups (p = 0.546). The criteria for optimal pacemaker performance was met in 749 (78%) patients, with similar rates in both age-groups (≥ 85 years vs < 85 years: odds ratio 1.03, 95%confidence interval 0.68–1.56, p = 0.888). A total of 119 complications occurred in 114 (12%) patients without significant age-group differences (p = 0.078). The most common complications were high ventricular lead thresholds/loss of conduction system capture (n = 58), pneumothorax (n = 13) and atrial lead dislodgements (n = 12) (Table 2). Of the 114 patients, 34 required reoperations for either lead revision or pacemaker extraction with similar rates in both age-groups (p = 0.389).
Table 3.
Comparison of pacing characteristics and complications.
| Total | < 85 years | ≥ 85 years | p value | |
|---|---|---|---|---|
| Pacing characteristics in whole cohort (n = 984) | ||||
| QRS duration baseline, ms | 102.6 ± 25.6 | 102.9 ± 25.6 | 101.2 ± 25.8 | 0.218 |
| QRS duration post-implant, ms | 118.6 ± 33.4 | 119.1 ± 33.7 | 115.7 ± 31.7 | 0.296 |
| Threshold at implant, V | 0.7 ± 0.4 | 0.7 ± 0.4 | 0.8 ± 0.4 | 0.256 |
| Threshold at follow-up, V | 0.9 ± 0.5 | 0.9 ± 0.5 | 1.0 ± 0.5 | 0.283 |
| R-wave at implant, mV | 10.5 ± 5.0 | 10.6 ± 5.0 | 9.7 ± 4.8 | 0.081 |
| R-wave at follow-up, mV | 11.2 ± 5.4 | 11.4 ± 5.4 | 10.2 ± 5.2 | 0.025 |
| Impedance at implant, ohm | 710.3 ± 184.7 | 714.9 ± 184.6 | 686.5 ± 183.8 | 0.049 |
| Impedance at follow-up, ohm | 534.8 ± 175.4 | 537.8 ± 186.2 | 519.1 ± 103.0 | 0.083 |
| Ventricular pacing percentage, % | 48.3 ± 44.5 | 46.2 ± 44.7 | 58.7 ± 41.9 | 0.002 |
| Pacing burden > 20% (%) | 546 (56) | 435 (53) | 111 (70) | < 0.001 |
| Pacing complications in whole cohort (n = 984) | ||||
| Complications, number of patients (%) | 114 (12) | 102 (12) | 12 (8) | 0.078 |
| Total number of complications, n | 119 | 107 | 12 | |
| Pneumothorax, n | 13 | 12 | 1 | |
| Vascular, n | 2 | 2 | 0 | |
| Abnormal ventricular lead thresholds/loss of HP capture, n | 58 | 51 | 7 | |
| Abnormal atrial lead thresholds, n | 2 | 2 | 0 | |
| Ventricular lead dislodgement, n | 5 | 5 | 0 | |
| Atrial lead dislodgement, n | 12 | 10 | 2 | |
| Wound defects, n | 9 | 8 | 1 | |
| Infection (pocket/endocarditis), n | 5 | 5 | 0 | |
| Pericardial effusion, n | 8 | 7 | 1 | |
| Interventricular septal injury, n | 4 | 4 | 0 | |
| Severe tricuspid regurgitation requiring left ventricular lead, n | 1 | 1 | 0 | |
| Reoperations for complications (%) | 34 (30) | 12 (36) | 7 (21) | 0.389 |
| Conduction system pacing (n = 462 attempts) | ||||
| Acute success (%) | 401 (87) | 325 (86) | 76 (88) | 0.632 |
| Pacing-complications (%) | 70 (17) | 62 (19) | 8 (11) | 0.077 |
| Optimal pacemaker performance (%) | 259 (65) | 208 (64) | 51 (69) | 0.461 |
| Absence of increased pacing threshold by > 1 V (%) | 363 (96) | 294 (96) | 69 (96) | 0.979 |
| Persistence of conduction system capture on follow-up (%) | 349 (87) | 280 (86) | 69 (91) | 0.279 |
Significant values are in [bold].
Secondary endpoints
Among 462 patients with attempted CSP, overall CSP success was 87% and did not differ by age (p = 0.632, Table 3). In 139 attempted HBP, 108 (78%) were successful, 7 converted to LBBP (5%) and 24 to RVP (17%), while in 323 attempted LBBP, 282 (87%) were successful, 4 converted to HBP (1%) and 37 (11%) to RVP. Of the final 401 patients with CSP (112 HBP and 289 LBBP), pacing-complications (19% vs 11%, p = 0.077), optimal pacemaker performance (64% vs 69%, p = 0.461), threshold stability (96% vs 96%, p = 0.979) and enduring conduction system capture on follow-up (86% vs 91%, p = 0.279), were similar in patients aged < 85 years compared to ≥ 85 years, respectively.
Discussion
Among patients with preserved LVEF and pacemakers indicated for bradycardia, CSP, compared to RVP, was associated with lower hazards of the primary endpoint of HF-hospitalizations, PCM requiring upgrade to CRT and all-cause mortality, driven primarily by a reduction of HF-hospitalizations. This prognostic benefit of CSP over RVP was independent of age. Similarly, age did not adversely affect the secondary endpoints of acute CSP success, pacing-complications, optimal pacemaker performance, threshold stability and enduring conduction system capture on follow-up. These findings suggest that CSP may be the preferred pacing modality of choice in very elderly patients ≥ 85 years to reduce adverse clinical events without compromised pacemaker performance and safety.
As the global population ages, the number of very elderly individuals living with pacemakers is expected to rise with increasing life expectancy. However, this group of vulnerable patients are often under-represented in clinical trials. In the Pacemaker Selection in the Elderly trial, “physiologic” pacing (dual-chamber) compared with ventricular pacing (single-chamber) improved quality of life but had no effect on the incidence of cardiovascular events or death7. In a large nationwide inpatient sample of > 115,000 patients, unadjusted mortality rates were 5.61% in those > 90 years, accompanied by longer length of stay and higher hospital costs8. Other cohort studies found that older patients and comorbidity burden were at increased risk of death, although mortality rates in elderly patients who received pacemakers were comparable to age- and sex-matched controls from the general population5,6,9–11. Age-related differences in pacemaker complications were heterogenous, but pneumothorax, pocket haematoma and lead dislodgements were the most commonly reported5,8,11,20. While these early studies performed 1–2 decades ago were focused primarily on mortality, age-mediated effects on clinical outcomes in patients physiological pacing in CSP compared to conventional RVP, optimal pacemaker performance derived from pacing characteristics regarded as key to preserving battery longevity and undersensing of intrinsic rhythms15, pacing complications, as well as CSP success rates and enduring conduction system capture remain unknown.
Findings from this study are consistent with earlier studies in demonstrating the higher risk of adverse clinical outcomes with increasing age, especially among very elderly patients ≥ 85 years. We extend current knowledge in establishing that the beneficial effects of CSP in reducing the risks of adverse clinical outcomes applied to even very elderly patients. Significantly, patients < 85 years and ≥ 85 years with CSP both demonstrated reductions in HF-hospitalizations, and trended towards improved survival, consistent with the beneficial clinical effects of CSP on all-comers reported in earlier studies12–14. This is likely accounted for by direct capture of the His-Purkinje system in CSP, affording greater physiological ventricular activation, increased electrical and mechanical synchrony, reduced LV dysfunction and remodeling, and greater haemodynamic benefit21–23. Accordingly, the greatest benefit of CSP was seen among those with a pacing burden of > 20%12,13, and would be particularly advantageous in the very elderly, as ventricular pacing burden was significantly higher in this group compared to younger patients. Additionally, given the known associations of increasing age with HF, arrhythmias, and PCM24,25, very elderly patients are at increased risks of pacing-associated morbidity, particularly as patients with pacemakers are now living longer, and more are presenting later in life with pacing requirements. CSP may therefore have an important role in reducing pacing-associated morbidity, recurrent HF-admissions, healthcare costs, worse comorbidity and quality of life in this vulnerable group of patients8.
In similar fashion, age had no adverse effect on pacing performance during follow-up. Although patients ≥ 85 years had lower R-wave amplitudes on follow-up, this was clinically insignificant (10.2 mV vs 11.4 mV) and differences in lead impedance at implant had abated on follow-up. Significantly, pacing metrics paramount to battery longevity were unaffected by age, with the very elderly ≥ 85 years just as likely as younger patients to achieve optimal pacemaker performance and pacing threshold stability. Likewise, complication rates were also not significantly higher among the very elderly unlike earlier studies8,20, with pneumothorax and lead dislodgements among the commonest. Major complications requiring repeat operations involving the entire pacemaker system did not differ significantly by age. The high number of abnormal ventricular pacing thresholds in this study were primarily due to the loss of conduction system capture on follow-up, occurring more frequently in younger patients than in those ≥ 85 years, which did not reach statistical significance. Among those whom CSP was attempted, acute success rates were high in the very elderly and were comparable to younger patients, as was threshold stability and enduring conduction system capture on follow-up. Additionally, the feasibility of crossing over between HBP and LBBP provides flexibility and greater options to the operator in the event of a failed initial CSP attempt without significant risks of increased complications in the very elderly. Taken together, CSP may be the preferred pacing modality of choice in this vulnerable group of very elderly patients in reducing adverse clinical outcomes, particularly as healthcare today shifts towards addressing the challenges of an aging population.
Limitations
Several limitations to this study should be acknowledged. First, results from this study were observational in nature. Future randomized controlled trials are urgently required to identify the optimal pacing modality in this group of very elderly patients with increasing life expectancy. Nonetheless, the comorbidity burden was largely similar between the age-groups, and a comprehensive adjustment of clinical risk factors had been performed to ascertain an age-related effect on clinical outcomes. Second, this study is not adequately powered to detect age-differences in clinical outcomes between HBP and LBBP. The optimal CSP-modality remains uncertain, although concerns regarding anatomical constraints, higher pacing thresholds and increased lead revisions have been associated with HBP15,26. A larger, adequately-sized sample of very elderly patients is required to study the optimal CSP-modality of choice, and the therapeutic and safety effects of CSP, in the very elderly. Third, lack in granularity on causes of death due to low autopsy rates for cultural reasons precluded analysis on the associations with cardiovascular/non-cardiovascular deaths. Finally, follow-up duration of CSP was shorter than RVP but early separation of Kaplan–Meier survival curves indicate a significant treatment effect with CSP12. Longer-term follow-up is required to determine the long-term safety and efficacy of CSP.
Conclusion
In this observational study, CSP was associated with reduced adverse clinical outcomes compared to RVP in patients with bradycardia pacing indications. Increasing age did not have a significant impact on the beneficial effects of CSP, and did not adversely affect pacemaker safety and performance. In the vulnerable group of very elderly patients with bradycardia, larger randomized studies are required to firmly establish CSP as be the preferred pacing modality of choice.
Supplementary Information
Acknowledgements
The authors thank all staff and patients at the National University Heart Center, Singapore, Ng Teng Fong General Hospital and Changi General Hospital diagnostic catheterization laboratories for their contributions.
Author contributions
E.T. wrote the main manuscript text and prepared the figures. R.S., J.Y.L., E.B., S.P.C., S.C.S., L.T., C.Y., V.H.T. and P.K. contributed to the main manuscript. All authors reviewed and edited the manuscript, and were involved in the execution of the study.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-69388-2.
References
- 1.Curtis, A. B., Karki, R., Hattoum, A. & Sharma, U. C. Arrhythmias in patients ≥ 80 years of age: Pathophysiology, management, and outcomes. J. Am. Coll. Cardiol.71, 2041–2057 (2018). 10.1016/j.jacc.2018.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan, E. S. J. & Lee, C.-H. Obstructive sleep apnea and arrhythmias in the elderly. Curr. Sleep Med. Rep.7, 197–205 (2021). 10.1007/s40675-021-00212-3 [DOI] [Google Scholar]
- 3.Vespa, J., Medina, L. & Armstrong, D. Demographic turning points for the United States: “Population projections for 2020 to 2060. Curr. Popul. Rep. US Census Bureau25, 1144 (2020). [Google Scholar]
- 4.Westaway, S. et al. Trends in the use, complications, and costs of permanent pacemakers in Australia: A nationwide study from 2008 to 2017. Pacing Clin. Electrophysiol.44, 266–273 (2021). 10.1111/pace.14161 [DOI] [PubMed] [Google Scholar]
- 5.Chao, T. F. et al. Long-term prognosis of patients older than ninety years after permanent pacemaker implantation: Does the procedure save the patients?. Can. J. Cardiol.30, 1196–1201 (2014). 10.1016/j.cjca.2014.04.010 [DOI] [PubMed] [Google Scholar]
- 6.Dang, D. et al. Procedural safety and long-term follow-up after pacemaker implantation in nonagenarians. Clin. Cardiol.41, 1315–1321 (2018). 10.1002/clc.23083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamas, G. A. et al. Quality of life and clinical outcomes in elderly patients treated with ventricular pacing as compared with dual-chamber pacing. Pacemaker selection in the elderly investigators. N. Engl. J. Med.338, 1097–1104 (1998). 10.1056/NEJM199804163381602 [DOI] [PubMed] [Google Scholar]
- 8.Mandawat, A., Curtis, J. P., Mandawat, A., Njike, V. Y. & Lampert, R. Safety of pacemaker implantation in nonagenarians: An analysis of the healthcare cost and utilization project-nationwide inpatient sample. Circulation127, 1453–1465 (2013). 10.1161/CIRCULATIONAHA.113.001434 [DOI] [PubMed] [Google Scholar]
- 9.Schmidt, B. et al. Pacemaker therapy in very elderly patients: long-term survival and prognostic parameters. Am. Heart J.146, 908–913 (2003). 10.1016/S0002-8703(03)00453-8 [DOI] [PubMed] [Google Scholar]
- 10.Shen, W. K. et al. Survival and functional independence after implantation of a permanent pacemaker in octogenarians and nonagenarians. A population-based study. Ann. Intern. Med.125, 476–480 (1996). 10.7326/0003-4819-125-6-199609150-00008 [DOI] [PubMed] [Google Scholar]
- 11.Udo, E. O. et al. Long-term outcome of cardiac pacing in octogenarians and nonagenarians. Europace14, 502–508 (2012). 10.1093/europace/eur329 [DOI] [PubMed] [Google Scholar]
- 12.Tan, E. S. J. et al. Clinical outcomes in conduction system pacing compared to right ventricular pacing in bradycardia. JACC Clin. Electrophysiol.9, 992–1001 (2023). 10.1016/j.jacep.2022.10.016 [DOI] [PubMed] [Google Scholar]
- 13.Abdelrahman, M. et al. Clinical outcomes of his bundle pacing compared to right ventricular pacing. J. Am. Coll. Cardiol.71, 2319–2330 (2018). 10.1016/j.jacc.2018.02.048 [DOI] [PubMed] [Google Scholar]
- 14.Sharma, P. S. et al. Clinical outcomes of left bundle branch area pacing compared to right ventricular pacing: Results from the Geisinger-Rush Conduction System Pacing Registry. Heart Rhythm19, 3–11 (2022). 10.1016/j.hrthm.2021.08.033 [DOI] [PubMed] [Google Scholar]
- 15.Tan, E. S. J. et al. Comparison of pacing performance and clinical outcomes between left bundle branch and his bundle pacing. JACC Clin. Electrophysiol.9, 1393–1403 (2023). 10.1016/j.jacep.2022.12.022 [DOI] [PubMed] [Google Scholar]
- 16.De Leon, J. et al. Adopting permanent His bundle pacing: Learning curves and medium-term outcomes. Europace24, 606–613 (2022). 10.1093/europace/euab278 [DOI] [PubMed] [Google Scholar]
- 17.Tan, E. S. J. et al. Use of extendable helix leads for conduction system pacing: Differences in lead handling and performance lead design impacts conduction system pacing. J. Cardiovasc. Electrophysiol.33, 1550–1557 (2022). 10.1111/jce.15528 [DOI] [PubMed] [Google Scholar]
- 18.Tan, E. S. J. et al. Conduction system versus biventricular pacing in heart failure with non-left bundle branch block. J. Cardiovasc. Electrophysiol.34, 976–983 (2023). 10.1111/jce.15881 [DOI] [PubMed] [Google Scholar]
- 19.Tan, E. S. J. et al. Simplifying follow-up of left bundle branch pacing leads: Assessment of left bundle branch capture using a programmer only. Heart Rhythm20, 777–778 (2023). 10.1016/j.hrthm.2023.01.031 [DOI] [PubMed] [Google Scholar]
- 20.Armaganijan, L. V. et al. Are elderly patients at increased risk of complications following pacemaker implantation? A meta-analysis of randomized trials. Pacing Clin. Electrophysiol.35, 131–134 (2012). 10.1111/j.1540-8159.2011.03240.x [DOI] [PubMed] [Google Scholar]
- 21.Catanzariti, D. et al. Permanent His-bundle pacing maintains long-term ventricular synchrony and left ventricular performance, unlike conventional right ventricular apical pacing. Europace15, 546–553 (2013). 10.1093/europace/eus313 [DOI] [PubMed] [Google Scholar]
- 22.Slotwiner, D. J. et al. Impact of physiologic pacing versus right ventricular pacing among patients with left ventricular ejection fraction greater than 35%: A systematic review for the 2018 ACC/AHA/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation140, e483–e503 (2019). 10.1161/CIR.0000000000000629 [DOI] [PubMed] [Google Scholar]
- 23.Tokavanich, N. et al. A network meta-analysis and systematic review of change in QRS duration after left bundle branch pacing, His bundle pacing, biventricular pacing, or right ventricular pacing in patients requiring permanent pacemaker. Sci. Rep.11, 12200 (2021). 10.1038/s41598-021-91610-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goyal, P. et al. Geriatric cardiology: Coming of age. JACC Adv.1, 100070 (2022). 10.1016/j.jacadv.2022.100070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ponnusamy, S. S., Syed, T. & Vijayaraman, P. Pacing induced cardiomyopathy: Recognition and management. Heart109, 1407–1415 (2023). 10.1136/heartjnl-2022-321723 [DOI] [PubMed] [Google Scholar]
- 26.Vijayaraman, P. et al. Permanent His-bundle pacing: Long-term lead performance and clinical outcomes. Heart Rhythm15, 696–702 (2018). 10.1016/j.hrthm.2017.12.022 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.