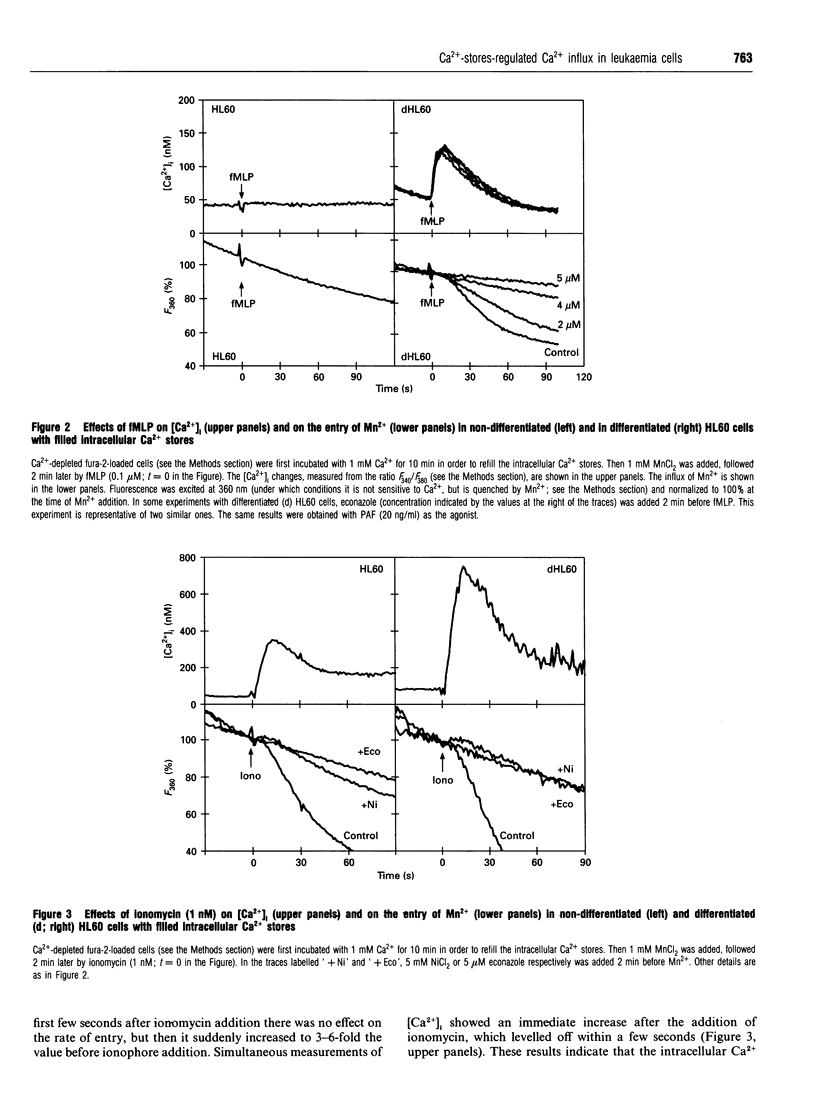
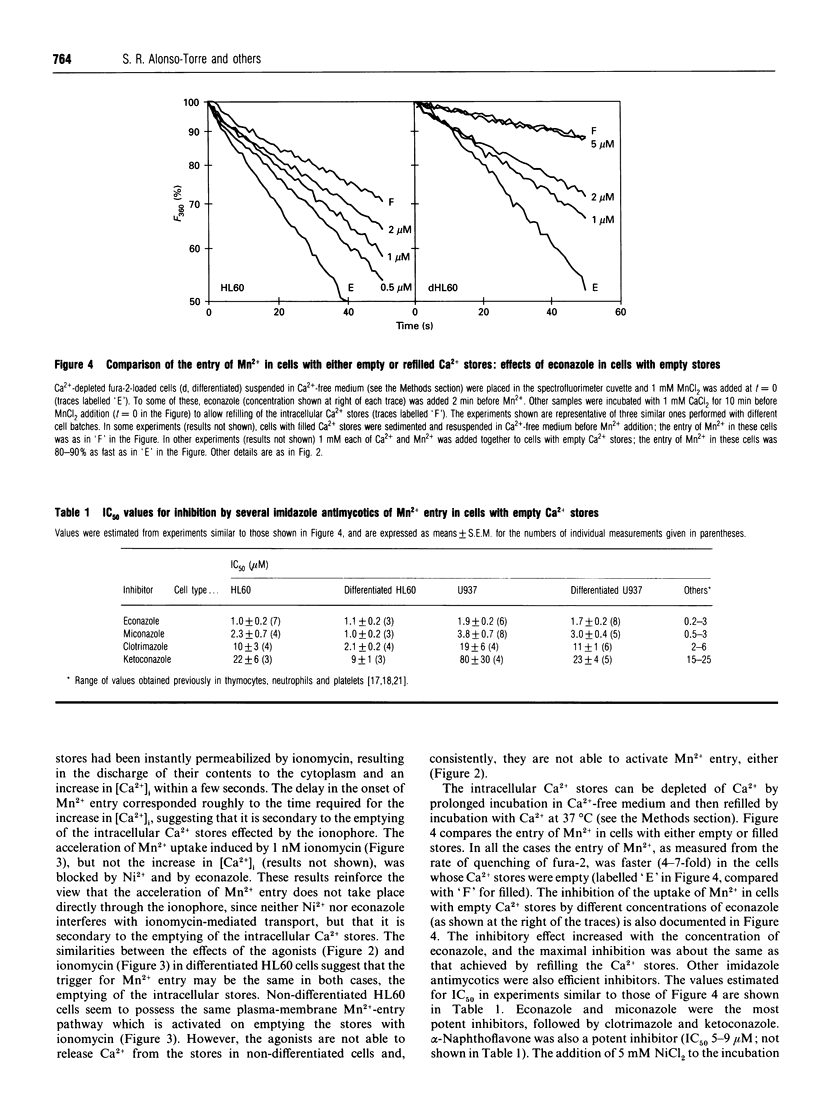
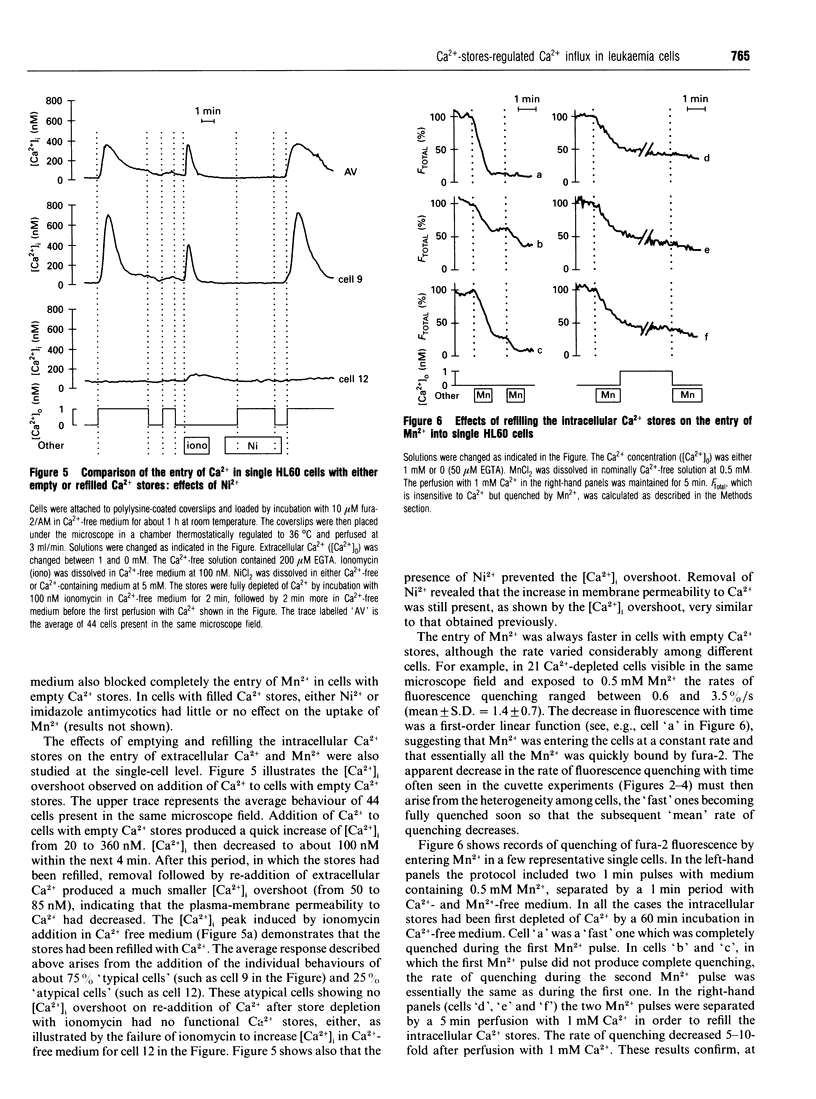
Abstract
Differentiation of HL60 cells by treatment with dimethyl sulphoxide induces the expression of membrane receptors for N-formylmethionyl-leucyl-phenylalanine (fMLP) and for platelet-activating factor (PAF). In these cells both agonists produced an increase in the cytosolic Ca2+ concentration ([Ca2+]i) by release of Ca2+ from the intracellular stores, followed shortly by an acceleration of the entry of Ca2+ or Mn2+, used here as a Ca2+ surrogate for Ca2+ channels. Cytochrome P-450 inhibitors blocked the agonist-induced entry of Ca2+ or Mn2+ with no modification of Ca2+ release from the stores. Emptying the intracellular Ca2+ stores either by treatments inducing no inositol phosphate production, such as prolonged incubation in Ca(2+)-free medium or treatment with the Ca2+ ionophore ionomycin, increased the plasma-membrane permeability to Ca2+ and Mn2+. This Ca(2+)-store-regulated Mn2+ entry was inhibited by Ni2+ and by cytochrome P-450 inhibitors. Refilling of the Ca2+ stores by incubation in Ca(2+)-containing medium restored low Mn2+ permeability. The same mechanism is present and functional in non-differentiated cells, before expression of membrane receptors for fMLP and PAF. These results suggest that agonist-induced Ca2+ (Mn2+) entry is secondary to the emptying of the intracellular Ca2+ stores, which in turn activates plasma-membrane channels by a mechanism involving cytochrome P-450.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert P. R., Tashjian A. H., Jr Ionomycin acts as an ionophore to release TRH-regulated Ca2+ stores from GH4C1 cells. Am J Physiol. 1986 Dec;251(6 Pt 1):C887–C891. doi: 10.1152/ajpcell.1986.251.6.C887. [DOI] [PubMed] [Google Scholar]
- Alonso M. T., Alvarez J., Montero M., Sanchez A., García-Sancho J. Agonist-induced Ca2+ influx into human platelets is secondary to the emptying of intracellular Ca2+ stores. Biochem J. 1991 Dec 15;280(Pt 3):783–789. doi: 10.1042/bj2800783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso M. T., Sanchez A., García-Sancho J. Monitoring of the activation of receptor-operated calcium channels in human platelets. Biochem Biophys Res Commun. 1989 Jul 14;162(1):24–29. doi: 10.1016/0006-291x(89)91956-6. [DOI] [PubMed] [Google Scholar]
- Alvarez J., García-Sancho J., Mollinedo F., Sanchez A. Intracellular Ca2+ potentiates Na+/H+ exchange and cell differentiation induced by phorbol ester in U937 cells. Eur J Biochem. 1989 Aug 15;183(3):709–714. doi: 10.1111/j.1432-1033.1989.tb21102.x. [DOI] [PubMed] [Google Scholar]
- Alvarez J., Montero M., Garcia-Sancho J. Cytochrome P450 may regulate plasma membrane Ca2+ permeability according to the filling state of the intracellular Ca2+ stores. FASEB J. 1992 Jan 6;6(2):786–792. doi: 10.1096/fasebj.6.2.1537469. [DOI] [PubMed] [Google Scholar]
- Alvarez J., Montero M., García-Sancho J. Cytochrome P-450 may link intracellular Ca2+ stores with plasma membrane Ca2+ influx. Biochem J. 1991 Feb 15;274(Pt 1):193–197. doi: 10.1042/bj2740193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artalejo A. R., García-Sancho J. Mobilization of intracellular calcium by extracellular ATP and by calcium ionophores in the Ehrlich ascites-tumour cell. Biochim Biophys Acta. 1988 Jun 7;941(1):48–54. doi: 10.1016/0005-2736(88)90212-x. [DOI] [PubMed] [Google Scholar]
- Ballard S. A., Lodola A., Tarbit M. H. A comparative study of 1-substituted imidazole and 1,2,4-triazole antifungal compounds as inhibitors of testosterone hydroxylations catalysed by mouse hepatic microsomal cytochromes P-450. Biochem Pharmacol. 1988 Dec 15;37(24):4643–4651. doi: 10.1016/0006-2952(88)90333-4. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Bird G. S., Rossier M. F., Hughes A. R., Shears S. B., Armstrong D. L., Putney J. W., Jr Activation of Ca2+ entry into acinar cells by a non-phosphorylatable inositol trisphosphate. Nature. 1991 Jul 11;352(6331):162–165. doi: 10.1038/352162a0. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Birnbaumer L. Direct G protein gating of ion channels. Am J Physiol. 1988 Mar;254(3 Pt 2):H401–H410. doi: 10.1152/ajpheart.1988.254.3.H401. [DOI] [PubMed] [Google Scholar]
- Capdevila J., Gil L., Orellana M., Marnett L. J., Mason J. I., Yadagiri P., Falck J. R. Inhibitors of cytochrome P-450-dependent arachidonic acid metabolism. Arch Biochem Biophys. 1988 Mar;261(2):257–263. doi: 10.1016/0003-9861(88)90340-2. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc Natl Acad Sci U S A. 1978 May;75(5):2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaurex N., Lew D. P., Krause K. H. Cyclopiazonic acid depletes intracellular Ca2+ stores and activates an influx pathway for divalent cations in HL-60 cells. J Biol Chem. 1992 Feb 5;267(4):2318–2324. [PubMed] [Google Scholar]
- Garcia-Sancho J., Alonso M. T., Sanchez A. Receptor-operated calcium channels in human platelets. Biochem Soc Trans. 1989 Dec;17(6):980–982. doi: 10.1042/bst0170980. [DOI] [PubMed] [Google Scholar]
- Garcia-Sancho J., Alvarez J., Montero M., Villalobos C. Ca2+ influx following receptor activation. Trends Pharmacol Sci. 1992 Jan;13(1):12–13. doi: 10.1016/0165-6147(92)90007-s. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hoth M., Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992 Jan 23;355(6358):353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- Hughes B. P., Crofts J. N., Auld A. M., Read L. C., Barritt G. J. Evidence that a pertussis-toxin-sensitive substrate is involved in the stimulation by epidermal growth factor and vasopressin of plasma-membrane Ca2+ inflow in hepatocytes. Biochem J. 1987 Dec 15;248(3):911–918. doi: 10.1042/bj2480911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F. 'Quantal' Ca2+ release and the control of Ca2+ entry by inositol phosphates--a possible mechanism. FEBS Lett. 1990 Apr 9;263(1):5–9. doi: 10.1016/0014-5793(90)80692-c. [DOI] [PubMed] [Google Scholar]
- Jacob R. Agonist-stimulated divalent cation entry into single cultured human umbilical vein endothelial cells. J Physiol. 1990 Feb;421:55–77. doi: 10.1113/jphysiol.1990.sp017933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P. S., Lin Y. J., Kao L. S. Caffeine-sensitive calcium stores in bovine adrenal chromaffin cells. J Neurochem. 1991 Jan;56(1):172–177. doi: 10.1111/j.1471-4159.1991.tb02577.x. [DOI] [PubMed] [Google Scholar]
- Lückhoff A., Clapham D. E. Inositol 1,3,4,5-tetrakisphosphate activates an endothelial Ca(2+)-permeable channel. Nature. 1992 Jan 23;355(6358):356–358. doi: 10.1038/355356a0. [DOI] [PubMed] [Google Scholar]
- Merritt J. E., Jacob R., Hallam T. J. Use of manganese to discriminate between calcium influx and mobilization from internal stores in stimulated human neutrophils. J Biol Chem. 1989 Jan 25;264(3):1522–1527. [PubMed] [Google Scholar]
- Missiaen L., Declerck I., Droogmans G., Plessers L., De Smedt H., Raeymaekers L., Casteels R. Agonist-dependent Ca2+ and Mn2+ entry dependent on state of filling of Ca2+ stores in aortic smooth muscle cells of the rat. J Physiol. 1990 Aug;427:171–186. doi: 10.1113/jphysiol.1990.sp018166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero M., Alvarez J., Garcia-Sancho J. Agonist-induced Ca2+ influx in human neutrophils is secondary to the emptying of intracellular calcium stores. Biochem J. 1991 Jul 1;277(Pt 1):73–79. doi: 10.1042/bj2770073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero M., Alvarez J., Garcia-Sancho J. Uptake of Ca2+ and refilling of intracellular Ca2+ stores in Ehrlich-ascites-tumour cells and in rat thymocytes. Biochem J. 1990 Oct 15;271(2):535–540. doi: 10.1042/bj2710535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naccache P. H., Molski T. F., Spinelli B., Borgeat P., Abboud C. N. Development of calcium and secretory responses in the human promyelocytic leukemia cell line HL60. J Cell Physiol. 1984 May;119(2):241–246. doi: 10.1002/jcp.1041190215. [DOI] [PubMed] [Google Scholar]
- Niedel J., Kahane I., Lachman L., Cuatrecasas P. A subpopulation of cultured human promyelocytic leukemia cells (HL-60) displays the formyl peptide chemotactic receptor. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1000–1004. doi: 10.1073/pnas.77.2.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandol S. J., Schoeffield M. S., Fimmel C. J., Muallem S. The agonist-sensitive calcium pool in the pancreatic acinar cell. Activation of plasma membrane Ca2+ influx mechanism. J Biol Chem. 1987 Dec 15;262(35):16963–16968. [PubMed] [Google Scholar]
- Pittet D., Lew D. P., Mayr G. W., Monod A., Schlegel W. Chemoattractant receptor promotion of Ca2+ influx across the plasma membrane of HL-60 cells. A role for cytosolic free calcium elevations and inositol 1,3,4,5-tetrakisphosphate production. J Biol Chem. 1989 May 5;264(13):7251–7261. [PubMed] [Google Scholar]
- Putney J. W., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986 Feb;7(1):1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Capacitative calcium entry revisited. Cell Calcium. 1990 Nov-Dec;11(10):611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- Ralph P., Williams N., Moore M. A., Litcofsky P. B. Induction of antibody-dependent and nonspecific tumor killing in human monocytic leukemia cells by nonlymphocyte factors and phorbol ester. Cell Immunol. 1982 Aug;71(2):215–223. doi: 10.1016/0008-8749(82)90257-x. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Sage S. O. Calcium signaling in human platelets. Annu Rev Physiol. 1990;52:431–449. doi: 10.1146/annurev.ph.52.030190.002243. [DOI] [PubMed] [Google Scholar]
- Sage S. O., Reast R., Rink T. J. ADP evokes biphasic Ca2+ influx in fura-2-loaded human platelets. Evidence for Ca2+ entry regulated by the intracellular Ca2+ store. Biochem J. 1990 Feb 1;265(3):675–680. doi: 10.1042/bj2650675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura H., Hughes A. R., Thastrup O., Putney J. W., Jr Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem. 1989 Jul 25;264(21):12266–12271. [PubMed] [Google Scholar]
- Vicentini L. M., Villereal M. L. Serum, bradykinin and vasopressin stimulate release of inositol phosphates from human fibroblasts. Biochem Biophys Res Commun. 1984 Sep 17;123(2):663–670. doi: 10.1016/0006-291x(84)90280-8. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Monck J. R. Hormone effects on cellular Ca2+ fluxes. Annu Rev Physiol. 1989;51:107–124. doi: 10.1146/annurev.ph.51.030189.000543. [DOI] [PubMed] [Google Scholar]


