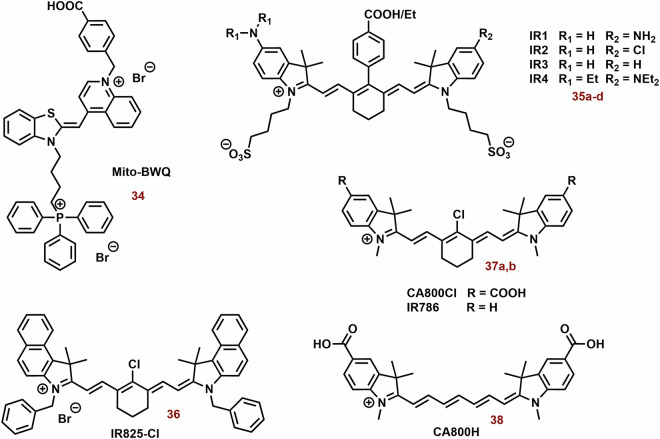
Fig. 13. The examples of photothermal cyanine dyes.
While heptamethine cyanine dyes are predominantly reported as PSs, triphenylphosphonium-substituted thiazole orange (34) has shown enhancements in photophysical properties and especially photothermal efficiency. Compounds 35a-d and 36 serve as intriguing examples of switchable PSs, demonstrating photothermal activity and near-infrared (NIR) fluorescence depending on pH and excitation wavelength, respectively. However, the impact of the cyanine dye structure on its tumor selectivity can be a crucial consideration. For instance, heptamethine 37a, featuring a chloro-cyclohexene ring and an indolium unit with a carboxyl group, exhibits significantly higher selectivity, possibly through interactions with serum albumin, compared to 37b (solely chloro-cyclohexene) and 38 (solely carboxylate groups).