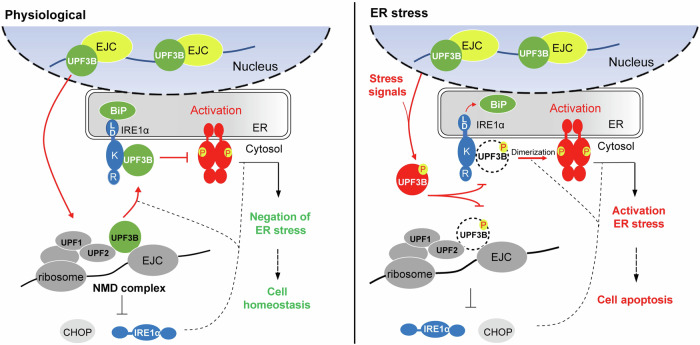
Fig. 8. The dual role of UPF3B in NMD and ER stress.
Under physiological conditions, UPF3B inhibits the activation of IRE1α and affects its phosphorylation and oligomerization by interacting with the IRE1α kinase domain. UPF3B and BiP jointly control the activation of IRE1α. In addition, NMD can inhibit ER stress by control the expression of IRE1α and CHOP, and negatively feedback ER stress to reshape cell homeostasis. During ER stress, UPF3B is phosphorylated and dissociates from IRE1α, which promotes the expression of IRE1α and CHOP, and activates ER stress, leading to apoptosis.