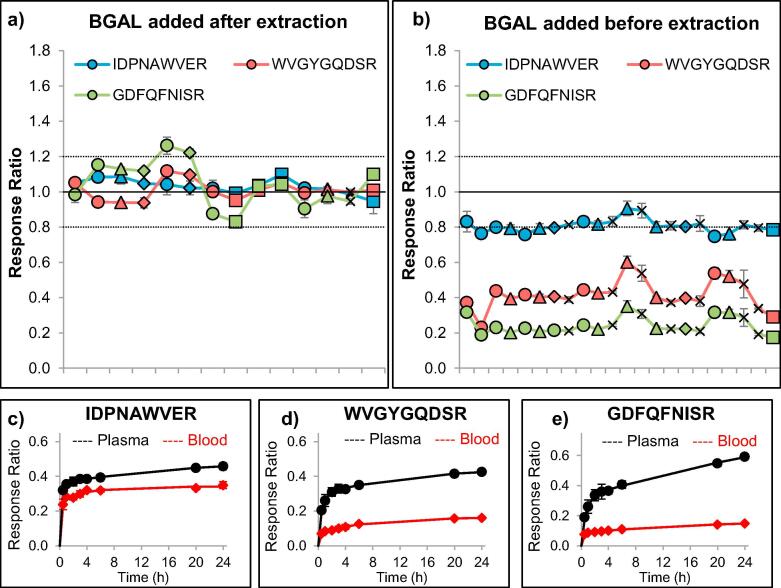
Fig. 2.
Optimization of the use of the exogenous protein beta-galactosidase (BGAL) as quality control for digestion efficiency among five blood specimens (indicated by different data symbols) at various time points (ordered chronologically with at least three analytical replicates at each time point). When the recombinant protein was spiked into the digestion buffer (after extraction, denaturation, reduction, and alkylation), the relative responses for three different peptides were consistently monitored around a 1:1 stoichiometry (a). When the recombinant protein was spiked into the extraction buffer (before extraction, denaturation, reduction, and alkylation), the relative peptide responses could still be consistently monitored, but did not provide an optimal 1:1 stoichiometry (b). Evaluation of the trypsin digestion efficiency of BGAL, which was spiked into plasma or blood before extraction, revealed a lower recovery of all three peptides in blood, compared to plasma (n = 3 at each time point). Of the three peptides, IDPNAWVER (c) showed the most-complete digestion, whereas WVGYGQDSR (d) and GDFQFNISR (e) did not reach more than 25% of the peptide reponse obtained after digestion in plasma.