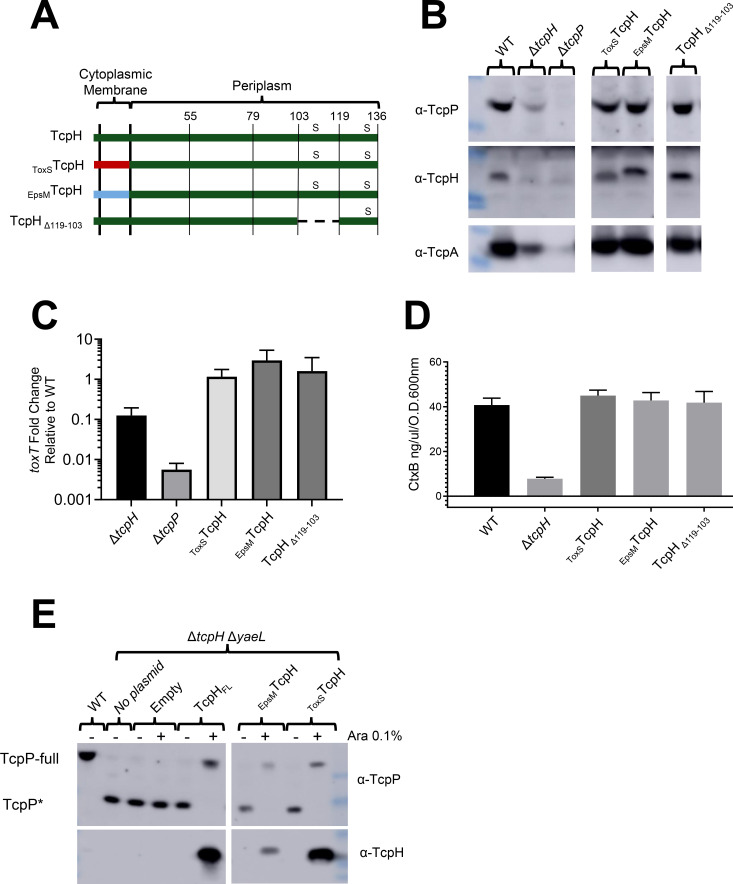
Fig 1.
TcpH transmembrane and periplasmic constructs protect TcpP, support toxT expression and virulence factor production. (A) Diagram of TcpH transmembrane constructs (EpsMTcpH and ToxSTcpH) and periplasmic construct (TcpH∆119-103). TcpH has a single transmembrane domain (also a Sec signal sequence), at its N-terminus, and two periplasmic cysteine residues (C114 and C132), represented by “s.” The transmembrane domain of TcpH was replaced with the transmembrane domain of ToxS (ToxSTcpH) and EpsM (EpsMTcpH) as both ToxS and EpsM are known to be localized to the cytoplasmic membrane with similar domain topology as TcpH (65, 66). In-frame deletion of periplasmic residues is indicated by a dashed line. (B-E) in vitro characterization of TcpH transmembrane and periplasmic chromosomal constructs grown under virulence-inducing conditions. Data presented in these panels were collected from three independent experiments. (B) Western blots of whole-cell lysates probed with α-TcpP (top), α-TcpH (middle), and α-TcpA (bottom). (C) Average toxT transcription of TcpH variants, determined via ∆∆CT method. toxT fold change is relative to WT V. cholerae. (D) CtxB levels, measured via enzyme-linked immunosorbent assay, in culture supernatants collected from cultures incubated with V. cholerae cells cultured in virulence-inducing conditions for 24 hours. Error bars represent the standard error of the mean. See Fig. S1B for the unmodified western blots in panel B. (C-D) Samples from independent experiments were averaged across three technical replicates. (E) Western blots of spheroplast fractions (cytoplasm and cytoplasmic membrane fractions). TcpH transmembrane constructs (ToxSTcpH and EpsMTcpH) and native TcpH were expressed from pBAD18 in ΔtcpH ΔyaeL background under virulence-inducing conditions for 6 hours. All strains, excluding WT, are ΔtcpH ΔyaeL.