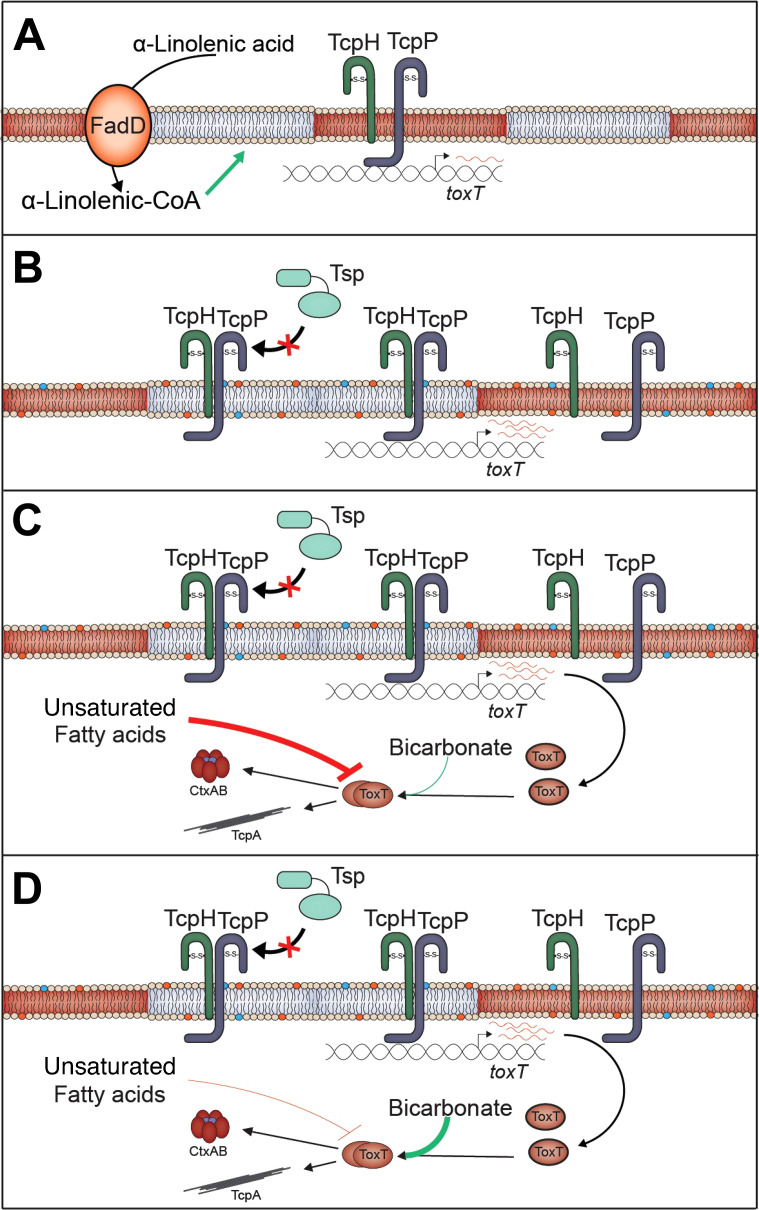
Fig 6.
α-Linolenic acid stimulates co-association of TcpP and TcpH within detergent-resistant membranes promoting TcpH-dependent inhibition of RIP. (A) Under virulence inducing (Vir Ind) conditions TcpP and TcpH molecules are associated with Triton insoluble (gray; TI) and Triton soluble (red; TS) membrane domains. (B) In the presence of exogenous α-linolenic acid, V. cholerae cells uptake α-linolenic acid (via VolA, FadL) and utilize it directly for phospholipid remodeling via the addition of coenzyme A (CoA), via FadD (67–70). This leads to changes in the overall phospholipid profile of V. cholerae, indicated by the blue and orange phospholipids (68–71). Under these conditions, a majority of TcpP and TcpH molecules transition to the TI membranes leading to enhanced inhibition of RIP by TcpH. The net result of α-linolenic acid supplementation is an increase in toxT transcription, indicated by an increase in red toxT mRNA. (C) In the lumen of the gastrointestinal tract, ToxT activity is thought to be inhibited by unsaturated fatty acids and thus inhibits ToxT-dependent virulence factor expression (75, 76, 131, 133–137). (D) Near the surface of epithelial cells, bicarbonate is actively secreted from intestinal epithelial cells and, as such, the concentration of bicarbonate is elevated, including in the crypt of intestinal villi (133–137). As bicarbonate can stimulate ToxT activity, ToxT-dependent virulence gene expression is proposed to be stimulated near epithelial cells (133–137).