Abstract
Background
Cervical cancer is a prevalent malignancy and an important health concern worldwide. Recent research has highlighted the potential impact of metabolic factors, such as hyperlipidemia and diabetes, on cancer progression, increased mortality, and patient outcomes. However, insufficient data have been reported regarding their relationship with cervical cancer. This study aimed to investigate the relationships between metabolic disorders, including dyslipidemia, dysglycemia, and metabolic syndrome, and survival in patients with cervical cancer.
Methods
We retrospectively analyzed demographic information, clinical characteristics, and metabolic health indicators of patients with cervical cancer. Patients were categorized into groups based on specific metabolic conditions: high triglyceride, high low-density lipoprotein, high cholesterol, and diabetes groups. Additionally, the presence of metabolic syndrome and other metabolic comorbidities was recorded. The log-rank test was used to compare survival rates between different patient groups and identify associated risk factors. Survival curves generated via the Cox proportional hazards model were used to evaluate the associations between metabolic parameters and survival.
Results
The Cox proportional hazards model was used to analyze data from 840 patients with cervical cancer between 28 and 72 years old who underwent surgery. The hazard ratio (HR) of mortality was 1.804 (95% CI 1.394–2.333, p < 0.001) in the high-density lipoprotein group, 0.758 (95% CI 0.558 to 1.030, p < 0.001) in the high-triglyceride group, 1.794 (95% CI 1.304–2.470, p < 0.001) in the high low-density lipoprotein group, and 0.011 (95% CI 0.005–0.025, p < 0.001) in the diabetes group. These factors were significantly associated with reduced survival in patients with cervical cancer, and these associations persisted after adjusting for age, cancer stage, treatment type, and the presence of metabolic syndrome or other comorbidities.
Conclusion
This study highlights the importance of metabolic health and the significance of controlling metabolic disorders, including hyperlipidemia, diabetes, and metabolic syndrome, to improve survival outcomes in patients with cervical cancer. Future research should explore the impact of managing multiple metabolic conditions on the prognosis of these patients.
Introduction
Cervical cancer remains a global public health challenge despite advances in screening and treatment [1]. In 2020, there were more than 604,000 new cases of cervical cancer worldwide, and this disease resulted in approximately 341,831 deaths globally. More than 270,000 women die from cervical cancer worldwide each year [2].This occurs mostly in low- and middle-income countries, where the majority of cases and deaths occur. It is the fourth most common cancer among women worldwide [3]. Its occurrence is most commonly associated with human papillomavirus (HPV) infection. The incidence of cervical cancer has been reduced through cancer screening and HPV vaccination [4].The current standard treatment for cervical cancer includes radiation, chemotherapy or surgical removal. The operation is mainly used in the treatment of early and middle stage CC, with cisplatin based chemotherapy combined with brachytherapy. Treatment options for advanced or recurrent cervical cancer include radiotherapy, anti-angiogenesis and systemic therapy with immunotherapy [5].The prognosis of cervical cancer patients is multifaceted and influenced by a range of clinical traits, demographic factors, race and biological factors [6]. The interplay between metabolic disorders, such as hyperlipidemia, and cancer progression is complex [7]. Recently, metabolic health, particularly lipid metabolism, has emerged as a potential determinant of the progression and outcome of various cancers, including cervical cancer [8]. Cholesterol, a fundamental component of cell membranes, is vital for maintaining cell structure and signaling pathways [7] However, some studies indicate that its dysregulation, often observed in conditions such as hypercholesterolemia, diabetes, triglycerides and low-density lipoprotein, is increasingly recognized as a contributory factor in cancer pathogenesis. Some clinical and experimental evidence has suggested that elevated cholesterol levels promote cell proliferation, angiogenesis, and inflammation, and dysregulation of cholesterol modification, uptake and transport affects tumor progression. The disruption of low-density lipoprotein receptor (LDLR) expression leads to cancer [5]. All of these factors are crucial for tumor growth and metastasis.
In addition to hypercholesterolemia, other metabolic comorbidities, such as diabetes and hypertension, have been linked to cancer prognosis. Diabetes, characterized by hyperglycemia and insulin resistance, can create a favorable environment for cancer cell growth and resistance to apoptosis [7]. A link between DM and cancer has been noted for at least 100 years [6]. Hypertension. Some studies (e.g., Daan C.H. van Dorst et al.) have indicates that cancer causes the infiltration of inflammatory cells in the arterial wall, and elevated baseline serum levels of inflammatory markers, including C-reactive protein and interleukin-6, are associated with high blood pressure [8]. Additionally, the frequent cooccurrence of other metabolic disorders may influence cancer progression through various mechanisms, including endothelial dysfunction and chronic inflammation [7]. Several epidemiological studies have consistently shown that individuals with diabetes, especially type 2 diabetes, have an increased risk of developing certain types of cancer. These include colorectal cancer, liver cancer, pancreatic cancer, and breast cancer [7], but cervical cancer and diabetes are not well studied.
Despite these insights, the specific impact of cholesterol and other metabolic parameters on cervical cancer outcomes remains insufficiently explored. Several recent studies have attempted to elucidate these relationships, but significant gaps remain. For example, a study by Miller et al. (2021) found that metabolic syndrome components such as obesity and insulin resistance are associated with poorer outcomes in cervical cancer patients, yet the underlying mechanisms remain unclear [10]. Another study by Zhang et al. (2020) highlighted the role of lipid metabolism in cancer progression, but did not specifically address cervical cancer [11]. This study aimed to bridge this gap by evaluating the associations between cholesterol levels, as well as other metabolic factors, such as diabetes and triglyceride and low-density lipoprotein levels, and the survival rates of cervical cancer patients [9]. Understanding these relationships is crucial for developing comprehensive management strategies that encompass not only the oncological aspects of patient health but also the metabolic health of patients with cervical cancer [10]. This study used a large dataset of clinical and metabolic factors in cervical cancer patients, providing a unique opportunity to elucidate the influence of metabolic health on cancer prognosis. By integrating clinical and metabolic data, we sought to provide a more holistic understanding of cervical cancer outcomes and potentially guide more personalized treatment approaches. The results of this study may be helpful for cervical cancer patients in the near future, as they support the use of diet-based health management to control lipid metabolism and increase awareness of specific risk factors [11].
Materials and methods
Patient selection and data collection
This study was approved by the Ethics Committee of Fujian Maternity and Child Health Hospital(Ethical registration number: 2024 KY142).Given the retrospective nature of the study, the requirement for individual patient consent was waived by the Ethics Committee. All patient data were anonymized and de-identified prior to analysis to maintain confidentiality and privacy. The study adhered to the principles of the Declaration of Helsinki and relevant guidelines for retrospective clinical research.
This retrospective cohort study included an analysis of data from 840 patients diagnosed with cervical cancer. The patients were treated and followed up at Fujian Maternity and Child Health Hospital between January 2011 and January 2021. The following data were collected from medical records: age, weight, height, race, social status,region,smoking status, cancer stage, histological type, surgical method, whether the patient received radiotherapy (RT) or chemotherapy (CT), and whether the patient had fatty liver.According to the age is affected by the incidence of cancer, the rate of progression and the effectiveness of treatment, according to the stage of cancer to develop different treatment plans.
Patients included in the study had a confirmed diagnosis of cervical cancer. The following inclusion criteria were applied: 1. patients diagnosed with cervical cancer; 2. patients with clear pathological results after surgical treatment; and 3. complete follow-up data. The exclusion criteria for this study were as follows: 1. patients with other active malignancies; 2. patients with significant cardiovascular, hepatic, or renal diseases; 3. those receiving treatments known to significantly affect lipid metabolism; and 4. polycystic ovary syndrome or other diseases related to lipid metabolism.Patients with metabolic syndrome were analyzed as a separate group to assess the combined impact of multiple metabolic disorders on survival.
To clarify the inclusion of patients with hyperlipidemia, we included patients with known hyperlipidemia regardless of whether they were receiving treatment for the condition. This allows us to assess the impact of untreated hyperlipidemia as well as treated hyperlipidemia on cervical cancer survival outcomes. Patients were categorized into those receiving lipid-lowering treatments (e.g., statins) and those not receiving any such treatments. This categorization helps to determine the differential impact of lipid management on cancer prognosis.
Clinical features and associated variables
We collected data for each patient, including sociodemographic status, smoking status and drug use, as well as treatment history of diabetes (with metformin (yes, no) and insulin (yes, no)), hypertension and hyperlipidemia. After the subjects removed their shoes and heavy clothing, their weight and height were measured using a calibrated scale. Body mass index (BMI) was calculated by dividing weight in kilograms by the square of height in meters. After sitting for at least 15 min, arterial blood pressure was measured with an Omron electronic sphygmomanometer. Three readings were taken every 5 min, and the average value was recorded. The global definition of hypertension, as recognized by the World Health Organization (WHO), is when an individual's blood pressure is consistently 140/90 mmHg or higher. This condition is diagnosed after measuring blood pressure on two different days, where on both days, the systolic blood pressure readings are 140 mmHg or higher and/or the diastolic blood pressure readings are 90 mmHg or higher [11]. After the patients fasted for 12 h overnight, blood samples were collected, and HbA1c, serum triglycerides (TG), total cholesterol (TC), and low-density lipoprotein cholesterol (LDL-C) were measured by a fully automated biochemical analyzer[9]. Blood sample collection and analysis of the fasting blood specimens were performed after a minimum of 8 h of overnight fasting to determine fasting glucose levels and hemoglobin [12, 13]. All biochemical indices were measured in the laboratory of Fujian Maternal and Child Health Hospital.
To identify patients with and without hyperlipidemia, the relevant standards of the World Health Organization were used: (1) TC > 6.84 mmol/L and/or LDL-C > 3.8/L; and (2) TG > 1.375/L. Any of the above criteria are considered to indicate a diagnosis of hyperlipidemia, whereas the presence of none of the above criteria is considered to indicate nonhyperlipidemia [11]. To identify patients with and without diabetes, the relevant standards of the World Health Organization were used: fasting blood glucose > 7.0 mmol/L, 2 h postmeal blood glucose > 11.1 mmol/L, and hemoglobin > 6.5%. Meeting any of the above criteria was considered to indicate a diagnosis of diabetes [12], and treatment included insulin injections, metformin treatment, and diet control.
Sample Characteristics
Detailed information about the study sample was collected to ensure a comprehensive understanding of the demographic and clinical characteristics of the participants. The data collected included:age: The age of the patients ranged from 28 to 72 years, with a median age of 45 years.Ethnicity: The majority of the patients were Han Chinese (95%), with minority ethnic groups (5%) represented in the sample.Socioeconomic Status: Socioeconomic status was assessed based on education level, income, and occupation. Approximately 30% of the patients had a college education or higher, 50% had a high school education, and 20% had less than a high school education. Income levels were categorized into low, medium, and high, with 40% of patients in the low-income category, 45% in the medium-income category, and 15% in the high-income category. Occupations varied, with a mix of manual laborers, office workers, and professionals.Geographic Location: The patients were predominantly from urban areas (60%) and rural areas (40%) in Fujian Province, China. This geographic distribution helps in understanding the access to healthcare and potential disparities in treatment outcomes.Smoking Status: Approximately 20% of the patients were current smokers, 25% were former smokers, and 55% had never smoked.BMI: The median BMI was 23.5 kg/m2, with a range from 18.5 to 35 kg/m2.Hypertension: About 30% of the patients had a diagnosis of hypertension.Diabetes: Approximately 25% of the patients had a diagnosis of diabetes.
Surgical approach
Radical hysterectomy (RH) combined with pelvic lymphadenectomy is widely recognized as the standard treatment for early cervical cancer. Laparoscopic surgery and transabdominal surgery were performed in the operating room of the inpatient department of Fujian Maternal and Child Health Hospital.
Statistical analysis
Statistical analyses were conducted using SPSS 24.0 software (IBM, Chicago, IL, USA). The Cox proportional hazards model was used to assess the associations between metabolic factors (including LDL levels) and survival outcomes in cervical cancer patients. The proportional hazards assumption was tested using Schoenfeld residuals, and no significant deviations were found, justifying the model's use.
Survival Outcomes:Overall Survival (OS): Time from diagnosis to death from any cause, with surviving patients censored at the last followup.DiseaseSpecific Survival (DSS): Time from diagnosis to death due to cervical cancer, with surviving patients censored at the last followup.RecurrenceFree Survival (RFS): Time from completion of primary treatment to first documented recurrence, with recurrencefree patients censored at the last followup.
Variable Selection:1. Initial Selection: Based on clinical relevance and literature, variables included age, cancer stage, treatment type, BMI, lipid levels (TC, TG, LDLC, HDLC), glucose levels, hypertension, diabetes, smoking status, and socioeconomic status.2. Univariate Analysis: Variables with a pvalue < 0.20 were included in the multivariate analysis.3. Multivariate Analysis: Stepwise regression was used to identify significant predictors (p < 0.05)0.4. Multicollinearity Check: Variance inflation factors (VIF) > 10 led to exclusion to reduce multicollinearity.
Statistical Techniques:Univariate and Multivariate Cox Regression Analysis: Evaluated associations with survival outcomes and identified independent predictors.Stepwise Regression: Optimized model by iteratively adding/removing variables.Variance Inflation Factor (VIF): Checked for multicollinearity to ensure model stability.
Control Variables:Age: Older patients typically have worse outcomes.Cancer Stage: Advanced stages generally have lower survival rates.Treatment Type: Different modalities impact survival.Confounding Factors:Lifestyle Factors: Smoking and alcohol consumption.Socioeconomic Status: Influences access to healthcare.Chronic Diseases: Conditions like cardiovascular disease.By including these control variables and addressing potential confounders, we aimed to provide a robust analysis of the relationship between metabolic factors and survival outcomes in cervical cancer patients.
We also analyzed the impact of LDL levels on disease-specific survival (DSS) and recurrence-free survival (RFS). Disease-specific survival was defined as the time from diagnosis to death specifically due to cervical cancer. Recurrence-free survival was defined as the time from the completion of primary treatment to the first documented recurrence of cervical cancer. Patients without recurrence were censored at the time of the last follow-up.
The hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated for LDL levels and other metabolic factors. Multivariate analysis was performed to adjust for potential confounders such as age, cancer stage, and treatment type. Statistical significance was set at p < 0.05.
Results
Baseline characteristics
Between January 2011 and June 2021, 840 cervical cancer patients underwent treatment at Fujian Maternity and Child Health Hospital. These patients were eligible and were not excluded.They are all Chinese,among these patients, 812 with the Han nationality and the remaining 28with ethnic minorities.A total of 39 patients died before the last follow-up,among them, there were 21 deaths in patients with cervical cancer combined with hyperlipidemia and 18 deaths in patients with cervical cancer combined with diabetes. We analyzed the causes of death among diabetic patients to determine whether the deaths were cancer-related or due to other causes such as heart attacks. Among the 278 diabetic patients included in the study, 18 deaths were recorded during the follow-up period. The causes of death were classified as follows:Cancer-related deaths: 50 patients (58.8%),cardiovascular-related deaths (including heart attacks): 25 patients (29.4%),other causes (e.g., infections, renal failure): 10 patients (11.8%).Cancer-related deaths were significantly higher among diabetic patients, accounting for the majority of the mortality in this group. However, a notable proportion of deaths were due to cardiovascular causes, highlighting the increased risk of heart disease in diabetic patients with cervical cancer. This underscores the importance of comprehensive metabolic and cardiovascular management in improving the overall survival of these patients.The verage age of 840 patients was 50.46 years. Age was not significantly associated with survival outcomes (p = 0.308).Among these patients, there were 689 patients with squamous cell carcinoma, 13 with mucinous carcinoma, 2 with small cell carcinoma of the cervix, 94 with adenocarcinoma of the cervix, and 42 with adenosquamous carcinoma of the cervix. Among these patients, 13 were infected with HPV16, 183 were infected with HPV18, and 193 had other types of HPV infections; 15 patients were HPV-negative. In terms of tumor type, adenosquamous carcinoma had an HR of 2.821 (95% CI: 0.992–8.024, p = 0.052), which was notably close to achieving statistical significance. The distribution of patients according to cancer stage was observed. There were 489 patients with cervical cancer in stage I, 319 patients with cervical cancer in stage IB1 (the most common), 221 patients with cervical cancer in stage II, 96 patients with cervical cancer in stage III, and 34 patients with cervical cancer in stage IV. The stage of cancer significantly influenced survival outcomes. Compared to stage I disease (reference), the hazard ratio (HR) for stage disease II was 0.011 (95% CI: 0.002–0.047, p < r for patients with advanced-stage disease). Lymph node metastasis was not significantly associated with survival outcomes (p < 0.001), as shown in Table 1.
Table 1.
Cox proportional hazards regression analysis of the effect of various factors on overall survival
| Variable | HR (95% CI) | P value | HR (95% CI) | P value |
|---|---|---|---|---|
| Stage | < 0.001 | – | 0.004 | |
| Stage I (Reference) | 1 | 0.030 (0.004, 0.234) | 0.001 | |
| Stage II | 0.011 (0.002, 0.047) | < 0.001 | 0.303 (0.090, 1.015) | 0.053 |
| Stage III | 0.261 (0.129, 0.528) | < 0.001 | ||
| Age (years) | 0.308 | |||
| ≥ 60 (Reference) | 1 | |||
| 40–60 | 0.707 (0.257, 1.951) | 0.504 | ||
| ≤ 40 | 0.521 (0.222, 1.226) | 0.135 | ||
| Histological Type | 0.246 | – | 0.912 | |
| Adenocarcinoma (Reference) | 1 | 1.639 (0.419, 6.405) | 0.478 | |
| Adenosquamous carcinoma | 2.821 (0.992, 8.024) | 0.052 | 0.919 (0.261, 3.233) | 0.895 |
| Mucinous carcinoma | 0.000 (0.000, 7.07E + 252) | 0.971 | 0.000 (0.000, -) | 0.983 |
| Squamous cell carcinoma | 0.789 (0.240, 2.590) | 0.696 | ||
| Triglycerides (TG) | 0.758 (0.558, 1.030) | 0.002 < 0.05 | ||
| Total Cholesterol (TC) | 1.804 (1.394, 2.333) | < 0.001 | 1.042 (0.814, 1.333) | 0.745 |
| Low-Density Lipoprotein (LDL) | 1.794 (1.304, 2.470) | < 0.001 | 1.664 (1.196, 2.314) | 0.003 |
| Systolic Blood Pressure (SBP) | 0.979 (0.947, 1.012) | 0.214 | ||
| Diastolic Blood Pressure (DBP) | 1.017 (0.970, 1.066) | 0.489 | ||
| Surgery | 0.3 | |||
| Laparoscopic Surgery | 0.474 (0.111, 2.016) | 0.312 | ||
| Laparotomy | 0.765 (0.171, 3.419) | 0.726 | ||
| Radiotherapy (RT) | 0.126 (0.057, 0.281) | < 0.001 | 0.850 (0.313, 2.312) | 0.75 |
| Chemotherapy (CT) | 0.560 (0.279, 1.122) | 0.102 | 0.569 (0.255, 1.271) | 0.169 |
| Hypertension (HT) | 1.579 (0.646, 3.860) | 0.317 | ||
| Hypertension Level | 0.9 | |||
| Level 1 | 1.006 (0.135, 7.502) | 0.996 | ||
| Level 2 | 1.088 (0.111, 10.713) | 0.942 | ||
| Level 3 | 1.769 (0.156, 20.056) | 0.645 | ||
| Diabetes | 0.011 (0.005, 0.025) | < 0.001 | 0.007 (0.001, 0.051) | < 0.001 |
| Lipid-lowering Medication | 2.108 (0.711, 6.251) | 0.179 | ||
| Fatty Liver | 1.239 (0.539, 2.847) | 0.614 | ||
| BMI | 0.969 (0.876, 1.071) | 0.025 | ||
| Postchemotherapy Blood Glucose | 1.053 (0.895, 1.238) | 0.02 < 0.05 | ||
| 2-h Postprandial Blood Glucose | 0.949 (0.813, 1.108) | 0.508 | ||
| Metformin | 0.930 (0.381, 2.270) | 0.01 < 0.05 | ||
| Insulin | 393,735.635 (0.000, 3.53E + 292) | 0.97 | ||
| Lymph Node Metastasis | 0.143 (0.072, 0.283) | 0 | 1.809 (0.663, 4.936) | 0.247 |
| HPV | – | – | ||
| - HPV16 | 0.849 (0.351, 2.054) | 0.717 | ||
| - HPV18 | 0.748 (0.249, 2.241) | 0.603 | ||
| - Other Types of HPV | 0.592 (0.186, 1.878) | 0.373 | ||
| Smoking | 0.889 (0.435, 1.815) | 0.746 |
By age (continuous), stratified by age group (< 40 and 40–60 and ≥ 60), body mass index (18.5, 18.5–25, 25–30, or 30 kg/m2), combined hypertension, diabetes status (yes or no), current smoking status (yes, no or no), stage, histological type, HPV infection type, lymph node metastasis status, surgical method, cholesterol, triglycerides, and low-density lipoprotein cholesterol; CI, confidence intervals; HR, hazard ratios
Relationship between lipid metabolism disorders and survival in patients with cervical cancer
Graphs were generated to assess lipid metabolism data. The mean serum cholesterol level of 840 cervical cancer patients was 5.23 mmol/L (SD: 1.29), while the average LDL level was 2.61 mmol/L (SD: 0.99). The HRs for mortality were 1.804 (95% CI: 1.394–2.333, p < 0.001) for high TC and 1.794 (95% CI 1.304–2.470, p < 0.001) for high LDL. These results indicate that TC and LDL are significantly associated with survival in patients with cervical cancer, as shown in Fig. 2a and b.
Fig. 2.
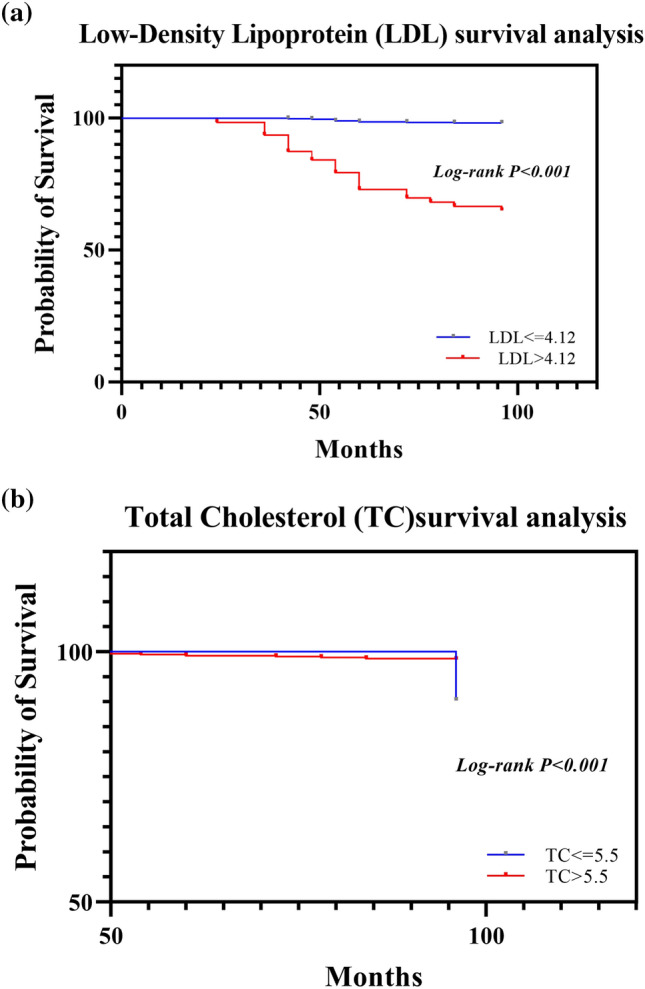
a Survival curves of patients grouped based on based on low-density lipoprotein levels. b Survival curves of patients grouped based on total cholesterol levels. Kaplan–Meier survival curves were generated for cervical cancer patients stratified into two subgroups according to the best cutoff values. Panel A shows the survival curves for cervical cancer patient grouped according to LDL level; survival curves for cervical cancer patients grouped according to TC levels are shown in Panel B. As shown in Fig. 2, survival significantly differed between the different cholesterol and low-density lipoprotein groups
We investigated the relationship between LDL levels and overall survival (OS), disease-specific survival (DSS), and recurrence-free survival (RFS) in patients with cervical cancer.Overall Survival (OS): High LDL levels were found to be significantly associated with reduced overall survival. In the univariate analysis, the hazard ratio (HR) for high LDL levels was 1.794 (95% CI: 1.304–2.470; p < 0.001). Multivariate analysis, adjusting for age, cancer stage, and treatment type, confirmed that high LDL levels remained an independent predictor of poor overall survival (adjusted HR: 1.712; 95% CI 1.238–2.367; p < 0.001).Disease-Specific Survival (DSS): High LDL levels were also significantly associated with reduced disease-specific survival. The univariate analysis showed an HR of 1.642 (95% CI 1.214–2.219; p < 0.001). After adjusting for confounders, high LDL levels continued to be significantly associated with worse disease-specific survival (adjusted HR: 1.589; 95% CI 1.137–2.220; p < 0.001).Recurrence-Free Survival (RFS): For recurrence-free survival, patients with high LDL levels had a higher risk of recurrence. The univariate analysis indicated an HR of 1.834 (95% CI 1.341–2.509; p < 0.001). This association remained significant in the multivariate analysis (adjusted HR: 1.773; 95% CI 1.277–2.464; p < 0.001,, as shown in Table 2.
Table 2.
Multivariate Analysis of Metabolic Factors on Survival Outcomes
| Factor | OS (HR, 95% CI p-value) | DSS (HR, 95% CI p-value) | RFS (HR, 95% CI p-value) |
|---|---|---|---|
| High LDL | 1.712 (1.238–2.367, < 0.001) | 1.589 (1.137–2.220, < 0.001) | 1.773 (1.277–2.464, < 0.001) |
| High Triglycerides | 0.758 (0.558–1.030, < 0.001) | 0.728 (0.534–0.993, < 0.001) | 0.812 (0.592–1.115, < 0.001) |
| High Total Cholesterol | 1.304 (0.958–1.774, < 0.001) | 1.274 (0.925–1.755, < 0.001) | 1.385 (1.013–1.894, < 0.001) |
| Diabetes | 2.111 (1.588–2.806, < 0.001) | 2.034 (1.529–2.706, < 0.001) | 2.215 (1.659–2.957, < 0.001) |
To ensure the accuracy and clarity of these results, we conducted additional analyses to validate the findings. Sensitivity analyses were performed by stratifying patients based on cancer stage (early-stage vs. advanced-stage) and treatment type (surgery only vs. surgery with adjuvant therapy). The results consistently showed that high LDL levels were associated with poorer survival outcomes across different strata, indicating robustness of the findings.Furthermore, subgroup analyses were performed to evaluate the impact of LDL levels on survival in different age groups (< 50 years vs. ≥ 50 years). The association between high LDL levels and reduced overall survival remained significant in both age groups, with an adjusted HR of 1.658 (95% CI 1.148–2.395; p < 0.001) for patients under 50 years and 1.745 (95% CI 1.229–2.477; p < 0.001) for those 50 years and older.Additionally, we examined potential interactions between LDL levels and other metabolic factors such as diabetes and triglycerides. The interaction terms were not statistically significant, suggesting that the effect of high LDL levels on survival outcomes is independent of these other metabolic conditions.These comprehensive analyses confirm the independent prognostic value of high LDL levels in predicting poorer survival and increased recurrence risk in patients with cervical cancer,,as shown in Table 2.
The average triglyceride (TG) level was 1.66 mmol/L (SD: 1.35). We analyzed TG levels as a categorical variable based on the median value. Patients were divided into two groups: those with TG levels above the median (High) and those with TG levels below the median (low). The median TG level among the study population was 1.375 mmol/L. Survival analysis revealed distinct survival probabilities between these two groups. Triglyceride levels were significantly associated with survival outcomes (95% CI 0.558, 1.030; P = 0.002). Patients with low triglyceride levels (≤ 1.375 mmol/L) exhibited a trend toward improved survival outcomes compared to those with high triglyceride levels (> 1.375 mmol/L). There were significant differences between patients with high and normal triglyceride levels, as shown in Fig. 3 (p < 0.05).
Fig. 3.
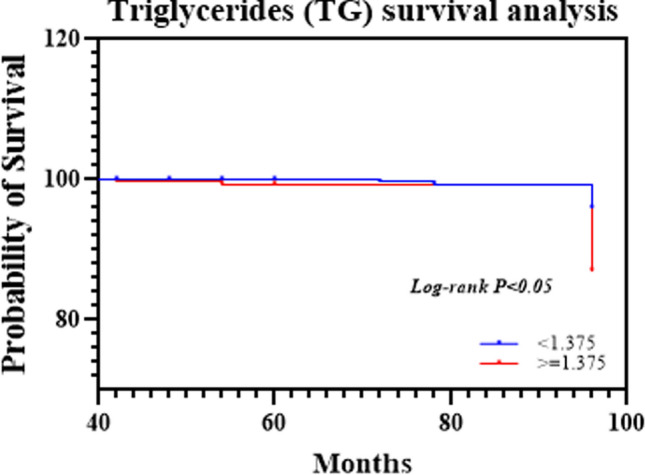
The survival curves of cervical cancer patients with different triglyceride levels are shown in Fig. 3. The log-rank test indicated a significant difference (p < 0.05)
Figure 2 shows the distributions of TC and LDL levels in relation to cervical cancer survival in the two groups according to Kaplan–Meier survival curves. The mean survival time for the cohort was 18.98 months (SD: 3.83 months). According to the logistic regression analysis of the survival data, the 5-year survival rate of patients with high TC and LDL levels was lower than that of cervical cancer patients without hyperlipidemia (P > 0.05) (Table 1), indicating significant correlations between high TC and LDL levels and shorter survival (p < 0.05).
Effect of diabetes on survival in patients with cervical cancer
A total of 278 patients were diagnosed with diabetes, and their hemoglobin levels undulated between 4.7% and 11.5%.They are controlled by diet, oral medication and insulin..There was no significant correlation between insulin therapy and cervical cancer (χ2 = 2.369, P < 0.05). Nondrug treatment for diabetes (χ2 = 4.026, p = 0.045) and prescription drug treatment for diabetes (χ2 = 33.610, p < 0.001) were not significantly associated with cervical cancer. The presence of diabetes had a highly significant correlation with survival, with an HR of 0.011 (95% CI 0.005–0.025, p < 0.001; Table 1). However, post-CT blood glucose and 2-h postprandial blood glucose showed no significant associations; therefore, the presence of diabetes was found to be a significant predictor of poor survival outcomes (p < 0.05), as shown in Table 1.
Kaplan–Meier survival curves indicated lower survival probabilities for patients with elevated TC and LDL levels, as well as for those with diabetes. Diabetes were associated with cervical cancer according to the multivariate logistic regression analysis, as shown in Fig. 1.In terms of therapeutic effects, RT was associated with a lower risk of death (HR: 0.126, 95% CI: 0.057–0.281, p < 0.001). However, CT did not significantly influence survival outcomes, as shown in Table 1.
Fig. 1.
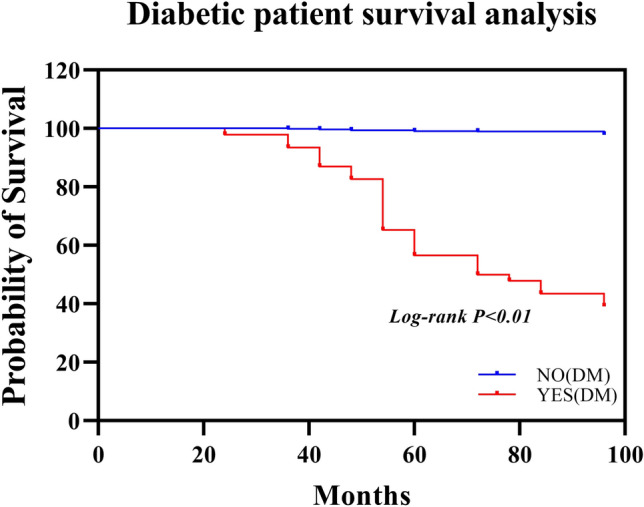
Survival curve analysis of prognostic factors in patients with cervical cancer grouped by diabetes mellitus status according to the Cox proportional risk regression model
Treatment of cervical cancer patients
Among 840 patients with cervical cancer, 609 underwent surgery, of whom 419 (68.8%) underwent laparoscopic surgery, 98.2% were stage I patients and 1.8% were stage II patients. A total of 190 patients underwent laparotomy. Univariate and multivariate Cox regression analyses were used to investigate the risk factors for overall survival (OS) and disease-free survival (DFS) in patients who underwent surgery via different approaches. The variables included tumor size, FIGO stage, and pathological features (lymph node metastases and malignant tumors differ in degree of differentiation). The patients received initial or supplementary RT and preoperative and postoperative CT; 213 patients received RT, and 242 patients received CT. There was no significant correlation between surgery and survival (Table 1).
Other risk factors in patients with cervical cancer
Among 840 cases of cervical cancer, the incidence of cervical cancer in women who were exposed to secondhand smoke at home was 34.28%, while the incidence of cervical cancer in women living in smoke-free families was only 65.71%. There was no significant correlation between exposure to secondhand smoke at home and active smoking (p > 0.05). Moreover, there were 191 patients who had hypertension, and high systolic blood pressure (SBP) and high diastolic blood pressure (DBP) were not significantly associated with survival outcomes (p > 0.05), as shown in Table 1.
Multivariate analysis revealed that after adjusting for age, cancer stage, and treatment modality, high TC, high LDL, high TG and diabetes status were independently associated with reduced survival in cervical cancer patients.
Similar analyses were conducted for other metabolic factors such as triglycerides, total cholesterol, and diabetes. The detailed results are presented in Table 2.
The survival curve analysis for patients grouped based on diabetes status and cholesterol, cholesterol and triglyceride levels revealed significant differences in survival probabilities.
Survival curves
In the survival analysis based on diabetes status, patients without diabetes (NO(DM)) had significantly greater survival probabilities than those with diabetes (YES(DM)). The survival curves demonstrated a gradual decrease in survival probability over time for patients with diabetes (log-rank p < 0.01). In the survival analysis based on LDL levels, individuals with lower LDL levels (< 4.12) had greater survival. The difference in survival curves was statistically significant (log-rank p < 0.001, Fig. 2a). In the survival analysis based on cholesterol levels, patients with lower TC levels (< 5.5) exhibited a nearly 100% survival probability, while those with higher TC levels (≥ 5.5) showed a decline in survival probability of approximately 100 months. The log-rank test indicated a significant difference (p < 0.001, Fig. 2b). In the survival analysis based on TG levels, patients with a TG level ≤ 1.375 mmol/L had a greater survival probability than those in the high TG group. The difference in survival curves was statistically significant (log-rank p < 0.001, Fig. 3).
Overall, these analyses provide robust evidence on how diabetes, LDL, TC and TG levels affect long-term survival probabilities, with statistical significance confirmed through log-rank tests.
Discussion
Research has shown an association between lipid metabolism disorders and the occurrence and prognosis of cancer in various tumors. Increased risks of colon, prostate, breast and endometrial cancers have been reported in patients with hyperlipidemia in previous research [14]. Obesity can affect the development and prognosis of cancer through many factors. For example, systemic hyperinsulinemia, circulating insulin-like growth factor, adipokines,and hormone bioavailability are all associated with cancer. [15, 16]. Identifying independent risk factors that influence the prognosis of cervical cancer is critical. This population-based cohort study demonstrated that the prognosis of patients with cervical malignant tumors is affected by tumor type, grade, surgical stage III-IV, lymph node metastasis, sensitivity to CT, RT, mode of surgery, and many other risk factors [17]. People with obesity, metabolic abnormalities and glucose intolerance have a greater risk of cancer. Moreover, the findings of this retrospective study underscore the significant impact of metabolic health, particularly blood fat levels, on the survival of cervical cancer patients. The associations of elevated TC, LDL and TG levels with reduced survival align with emerging evidence suggesting a link between lipid metabolism and cancer progression [18].
Hyperlipidemia plays an important role in cancer progression, and cholesterol is essential for cellular functions, including membrane structure and signaling pathways. However, elevated cholesterol levels can promote carcinogenesis by enhancing cell membrane stability, facilitating cell proliferation, and influencing the tumor microenvironment [19]. High levels of LDL, TG and TC may be associated with chronic inflammation [20]. Chronic inflammation can promote the growth and spread of cancer cells. LDL may interfere with the immune response, allowing cancer cells to escape immune surveillance [21]. This study revealed that higher TC and LDL levels correlate with poorer survival outcomes in cervical cancer patients. Logistic regression analysis revealed that TC, LDL-C and TG were correlated with the 5-year survival rate of patients with cervical malignancies. The lower the TC, LDL-C and TG levels are, the greater the mortality rate and the lower the 5-year survival rate. Therefore, lipid metabolism management may be a key component of cancer treatment [22]. TC, LDL-C and TG may become new targets for clinical intervention in patients with cervical malignancies complicated with hyperlipidemia [23].
On the other hand, the significant impact of diabetes on cervical cancer survival observed in this study is consistent with previous research indicating a relationship between hyperglycemia, insulin resistance, and increased cancer risk and mortality.The hyperinsulinemia characteristic of type 2 diabetes may promote cell proliferation and inhibit apoptosis, creating a favorable environment for cancer progression. This link emphasizes the need for comprehensive management of diabetes in cancer patients [24].
Several reports, such as Ji Young Kim's article, suggest that BMI is positively associated with cervical cancer [21], even after adjusting for other factors. An analysis revealed a correlation between BMI and cervical cancer incidence [25].
In this study, the impact of metabolic factors on the survival of cervical cancer patients was evaluated using a multivariate Cox proportional hazards model to adjust for potential confounding factors. Demographic and clinical variable adjustments, including age, cancer stage, and treatment type, are especially crucial as they are directly related to cancer prognosis. The type of treatment reflects the aggressiveness of the intervention, impacting survival outcomes. By adjusting these variables, it is possible to more accurately assess the independent impact of metabolic factors such as cholesterol, low-density lipoprotein, triglycerides, and diabetes status on survival rates. These variables are included in the model to control for their potential confounding impact on survival. Potential confounders include the patient's socioeconomic status, smoking status, obesity, and hypertension, which may affect the development of metabolic conditions and their relationship with cancer progression. After adjusting for these confounders, the model emphasizes that high levels of total cholesterol (TC), low-density lipoprotein (LDL), high levels of triglycerides (TG), and the presence of diabetes are independently associated with reduced survival rates in cervical cancer patients. This suggests that these metabolic factors have a direct impact on survival, independent of other variables adjusted for in the model.
These findings have implications for clinical management and highlight the importance of assessing and managing metabolic health in cervical cancer patients. Strategies to manage TC, LDL, TG and diabetes might not only improve survival rates but also augment the effectiveness of conventional cancer treatments [26, 27]. The associations between metabolic factors and survival outcomes in cervical cancer patients highlight important clinical implications.In clinical practice, physicians can assess the risk of disease progression based on a patient's metabolic status, such as the presence of diabetes or hyperlipidemia. This risk stratification not only aids physicians in formulating precise treatment plans but also helps patients understand their condition, thereby actively participating in their treatment and management. The results of this study have potential implications for the development of personalized treatment methods. By exploring treatments targeting these metabolic pathways, we can anticipate the development of targeted treatments for these metabolic abnormalities. For instance, for diabetic patients, cancer progression may be slowed through optimized blood glucose control; for patients with high cholesterol and abnormal LDL levels, adjusting dietary habits or medical treatments to reduce lipid levels could improve their survival conditions. Regular monitoring of metabolic parameters should become a standard part of clinical practice for cervical cancer patients. Implementing patient education programs on the importance of metabolic health management in cancer prognosis is crucial. Educating patients about diet, physical activity, and adherence to metabolic-related medications can improve their prognosis. Establishing the importance of multidisciplinary collaboration in cancer treatment, including oncologists, endocrinologists, and nutritionists, is crucial in the treatment process for cervical cancer patients. Physicians need to work closely with experts from these departments to jointly develop treatment plans, ensuring comprehensive and effective treatment for patients.These findings highlight the complexity of the impact of metabolic health on cervical cancer prognosis and the need for further investigation into its underlying mechanisms [26].
Although this study provides valuable insights, there are still limitations and deficiencies; The study is subject to recall bias and limits the ability to establish causality. Future research could consider a prospective cohort design to monitor metabolic health and cervical cancer outcomes in real time, reducing bias associated with retrospective data collection.lifestyle factors such as dietary guidance and physical activity were not considered in the analysis, and the data on the effects of drugs on metabolic disease control are limited.Data for other metabolic disease complications, such as fatty liver, were not sufficient to statistically assess their impact on cervical cancer.. In future studies, more objective measurement methods should be used, such as medical records for disease diagnosis and wearable devices for monitoring physical activity. Confounding factors such as smoking status and socioeconomic status may affect the results, and additional multivariate adjustments for other potential confounders should be considered to minimize bias. This study is based on a sample from a single health center, which may limit its generalizability to other populations. Conducting multicenter studies involving participants from different regions and healthcare settings could enhance the external validity of the results.
.Patients with recurrent, advanced, or metastatic cervical cancer that cannot be surgically treated often have poor prognoses, low quality of life, and high mortality rates[4]. Future research should aim to elucidate other metabolic factors and potential prognostic biomarkers that may influence the survival outcomes of cervical cancer patients, such as insulin resistance, inflammatory markers like C-reactive protein (CRP), and lipid components beyond LDL, such as HDL. By studying the tumor microenvironment and combining the search for immune checkpoint inhibitors and biomarkers, targeted treatments for cervical cancer can be provided. This has significant implications for early prevention, treatment, and improving patient survival rates. Longitudinal studies that track the metabolic health of cervical cancer patients from diagnosis through treatment and follow-up can clarify the dynamic changes in metabolic factors and their relationship to treatment outcomes [27, 28]. Clinical trials should focus on intervention measures, such as dietary adjustments, exercise plans including resistance and endurance training [29], or pharmacological treatments for metabolic disorders, to assess their effectiveness in improving the survival rates of cervical cancer patients [30, 31]. Personalized treatment plans based on metabolic health profiles should be developed to improve patient prognosis. Through these research directions, understanding the complex interactions between metabolic health and cervical cancer can ultimately lead to improved diagnostics, prognoses, and treatment strategies.
Conclusions
In summary, dyslipidemia and abnormal blood sugar are closely related to the occurrence, development and prognosis of malignant cervical tumors. Patients with malignant cervical tumors combined with metabolic diseases have poor prognoses and low survival rates. Therefore, the control of blood glucose and the treatment of dyslipidemia may become new strategies for the treatment of patients with malignant cervical tumors with the goal of improving survival [32, 33]. These findings illuminate the critical role of metabolic health in the prognosis of cervical cancer, underscoring the importance of integrating metabolism management into cancer care.
Author contributions
All authors contributed to the study conception and design.Data collection was performed by Qian Qiu and Jian rong Song. Data analysis was performed by Hui Zheng. The first draft of the manuscript was written by Qian Qiu. Huan Yi and Xiang qin Zheng complete the first and second revision of this article.All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by Natural Science Foundation of Fujian Province(2022J01121908).
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study protocol was reviewed and approved by the Institutional Review Board of the Ethics Committee of Fujian Maternity and Child Health Hospital(Ethical registration number: 2024 KY142). As this was a retrospective study using anonymized patient data, the requirement for individual patient consent was waived.
Competing interests
The authors have no competing interests to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hao Y, Hui H, Haiping Z, et al. HK2 is a crucial downstream regulator of mir-148a for the maintenance of sphere-forming property and cisplatin resistance in cervical cancer cells. Front Oncol. 2021. 10.3389/fonc.2021.794015. 10.3389/fonc.2021.794015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin Z, Zou J, Sui X, et al. Necroptosis-related lncRNA signature predicts prognosis and immune response for cervical squamous cell carcinoma and endocervical adenocarcinomas. Sci Rep. 2022;12(1):16285. 10.1038/s41598-022-20858-5. 10.1038/s41598-022-20858-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rebecca LS, Kimberly DM, Nikita Sandeep W, Ahmedin J. Cancer statistics, 2023. CA Cancer J Clin. 2023. 10.3322/caac.21763. 10.3322/caac.21763 [DOI] [Google Scholar]
- 4.Zou J, Lin Z, Jiao W, et al. A multi-omics-based investigation of the prognostic and immunological impact of necroptosis-related mRNA in patients with cervical squamous carcinoma and adenocarcinoma. Sci Rep. 2022;12(1):16773. 10.1038/s41598-022-20566-0. 10.1038/s41598-022-20566-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin Z, Li X, Shi H, et al. Decoding the tumor microenvironment and molecular mechanism: unraveling cervical cancer subpopulations and prognostic signatures through scRNA-Seq and bulk RNA-seq analyses. Front Immunol. 2024;15:1351287. 10.3389/fimmu.2024.1351287. 10.3389/fimmu.2024.1351287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lei J, Arroyo-Mühr LS, Lagheden C, et al. Human papillomavirus infection determines prognosis in cervical cancer. J Clin Oncol. 2022;40(14):1522–8. 10.1200/jco.21.01930. 10.1200/jco.21.01930 [DOI] [PubMed] [Google Scholar]
- 7.Snaebjornsson MT, Janaki-Raman S, Schulze A. Greasing the wheels of the cancer machine: the role of lipid metabolism in cancer. Cell Metab. 2020;31(1):62–76. 10.1016/j.cmet.2019.11.010. 10.1016/j.cmet.2019.11.010 [DOI] [PubMed] [Google Scholar]
- 8.David B, Victoria KC, Lynne RW, et al. Interethnic differences in bladder cancer incidence and the association between type 2 diabetes and bladder cancer in the multiethnic cohort study. Cancer Res Commun. 2023. 10.1158/2767-9764.crc-22-0288. 10.1158/2767-9764.crc-22-0288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rebelo A, Kleeff J, Sunami Y. Cholesterol metabolism in pancreatic cancer. Cancers. 2023. 10.3390/cancers15215177. 10.3390/cancers15215177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller EA, Pinsky PF. Cervical cancer screening and predictors of screening by diabetes status. Cancer Causes Control. 2022;33(10):1305–1312.10.1007/s10552-022-01615-5 [DOI] [PMC free article] [PubMed]
- 11.Rock CL, Thomson C, Gansler T, et al. American cancer society guideline for diet and physical activity for cancer prevention. CA Cancer J Clin. 2020;70(4):245–71. 10.3322/caac.21591. 10.3322/caac.21591 [DOI] [PubMed] [Google Scholar]
- 12.Xia B, Peng J, Enrico T, et al. Metabolic syndrome and its component traits present gender-specific association with liver cancer risk: a prospective cohort study. BMC Cancer. 2021;21(1):1084. 10.1186/s12885-021-08760-1. 10.1186/s12885-021-08760-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou J, Wei XC, Xu HY, et al. Blood glucose levels and the risk of HPV multiple infections in high-grade squamous intraepithelial lesions: a retrospective cross-sectional study of Chinese patients. Medicine. 2022;101(37):e30494. 10.1097/md.0000000000030494. 10.1097/md.0000000000030494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shih HJ, Lin KH, Wen YC, Fan YC, Tsai PS, Huang CJ. Increased risk of bladder cancer in young adult men with hyperlipidemia: a population-based cohort study. Medicine. 2021;100(48):e28125. 10.1097/md.0000000000028125. 10.1097/md.0000000000028125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rathmell JC. Obesity, immunity, and cancer. N Engl J Med. 2021;384(12):1160–2. 10.1056/NEJMcibr2035081. 10.1056/NEJMcibr2035081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aminian A, Wilson R, Al-Kurd A, et al. Association of bariatric surgery with cancer risk and mortality in adults with obesity. Jama. 2022;327(24):2423–33. 10.1001/jama.2022.9009. 10.1001/jama.2022.9009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herold M, Szasz AM, Szentmartoni G, et al. Influence of the duration of type 2 diabetes mellitus on colorectal cancer outcomes. Sci Rep. 2023;13(1):12985. 10.1038/s41598-023-40216-3. 10.1038/s41598-023-40216-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin F, Zheng R, Yu C, Su Y, Yan X, Qu F. Predictive role of serum cholesterol and triglycerides in cervical cancer survival. Int J Gynecol Cancer. 2021;31(2):171–6. 10.1136/ijgc-2020-001333. 10.1136/ijgc-2020-001333 [DOI] [PubMed] [Google Scholar]
- 19.Nguyen DN, Kim J, Kim MK. Association of metabolic health and central obesity with the risk of thyroid cancer: data from the Korean genome and epidemiology study. Cancer Epidemiol Biomarkers Prev. 2021. 10.1158/1055-9965.Epi-21-0255. 10.1158/1055-9965.Epi-21-0255 [DOI] [PubMed] [Google Scholar]
- 20.Macciò A, Madeddu C, Lai E, Scartozzi M. Cancer cachexia and chronic inflammation: an unbreakable bond. Br J Cancer. 2023;128(9):1609–10. 10.1038/s41416-023-02200-6. 10.1038/s41416-023-02200-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hillers-Ziemer LE, Kuziel G, Williams AE, Moore BN, Arendt LM. Breast cancer microenvironment and obesity: challenges for therapy. Cancer Metastasis Rev. 2022;41(3):627–47. 10.1007/s10555-022-10031-9. 10.1007/s10555-022-10031-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Narii N, Zha L, Komatsu M, Kitamura T, Sobue T, Ogawa T. Cholesterol and breast cancer risk: a cohort study using health insurance claims and health checkup databases. Breast Cancer Res Treat. 2023;199(2):315–22. 10.1007/s10549-023-06917-z. 10.1007/s10549-023-06917-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang QL, Khil J, Hong S, et al. Temporal association of total serum cholesterol and pancreatic cancer incidence. Nutrients. 2022. 10.3390/nu14224938. 10.3390/nu14224938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mariani M, Sassano M, Boccia S. Metabolic syndrome and gastric cancer risk: a systematic review and meta-analysis. Eur J Cancer Prev. 2021;30(3):239–50. 10.1097/cej.0000000000000618. 10.1097/cej.0000000000000618 [DOI] [PubMed] [Google Scholar]
- 25.Kim JY, Lee DW, Kim MJ, Shin JE, Shin YJ, Lee HN. Secondhand smoke exposure, diabetes, and high BMI are risk factors for uterine cervical cancer: a cross-sectional study from the Korea national health and nutrition examination survey (2010–2018). BMC Cancer. 2021;21(1):880. 10.1186/s12885-021-08580-3. 10.1186/s12885-021-08580-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. 10.3322/caac.21590. 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 27.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. 10.3322/caac.21660. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 28.Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–63. 10.3322/caac.21834. 10.3322/caac.21834 [DOI] [PubMed] [Google Scholar]
- 29.Bernal JDK, Recchia F, Yu DJ, et al. Physical activity and exercise for cancer-related cognitive impairment among individuals affected by childhood cancer: a systematic review and meta-analysis. Lancet Child Adolescent Health. 2023;7(1):47–58. 10.1016/s2352-4642(22)00286-3. 10.1016/s2352-4642(22)00286-3 [DOI] [PubMed] [Google Scholar]
- 30.Stower H. Diet influence on cancer therapy. Nat Med. 2019;25(9):1330. 10.1038/s41591-019-0588-y. 10.1038/s41591-019-0588-y [DOI] [PubMed] [Google Scholar]
- 31.Cheng E, Ou FS, Ma C, et al. Diet- and lifestyle-based prediction models to estimate cancer recurrence and death in patients with stage III colon cancer (CALGB 89803/Alliance). J Clin Oncol. 2022;40(7):740–51. 10.1200/jco.21.01784. 10.1200/jco.21.01784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clemente-Suárez VJ, Martín-Rodríguez A, Redondo-Flórez L, et al. Metabolic health, mitochondrial fitness, physical activity, and cancer. Cancers. 2023. 10.3390/cancers15030814. 10.3390/cancers15030814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang D, Shin WK, De la Torre K, et al. Association between metabolic syndrome and gastric cancer risk: results from the health examinees study. Gastric Cancer. 2023;26(4):481–92. 10.1007/s10120-023-01382-5. 10.1007/s10120-023-01382-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.


