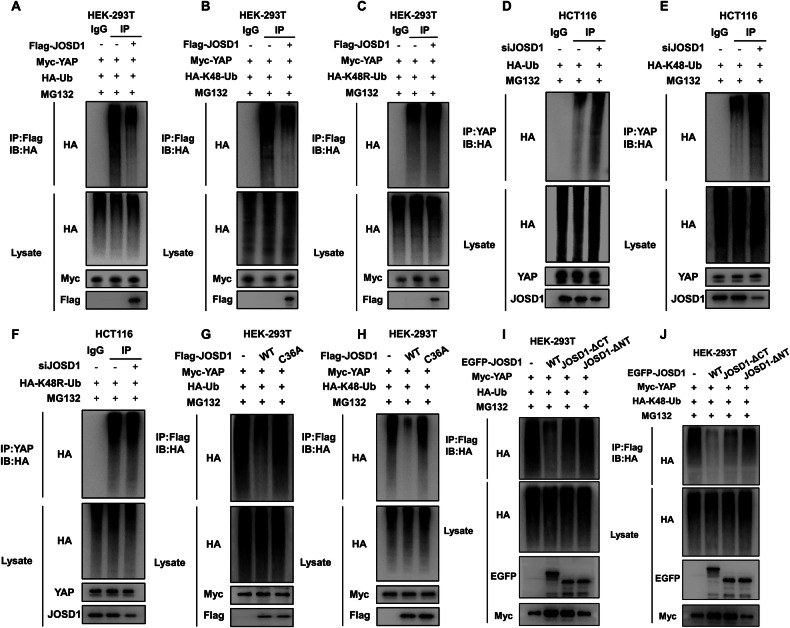
Fig. 6. JOSD1 stabilizes YAP via inhibiting YAP K48-linked poly-ubiquitination.
A JOSD1 decreased YAP polyubiquitination. After treating HEK-293T cells with MG132 for 6 h, they were transfected with 2 μg of YAP plasmid, 0.5 μg of HA-Ub plasmid, and either 0.5 μg of Flag-tag or Flag-JOSD1 plasmids. Subsequently, the cells were immunoblotted with specific antibodies. Polyubiquitination of YAP was reduced by JOSD1. B, C JOSD1 decreased YAP polyubiquitination. After treating HEK-293T cells with MG132 for 6 h, they were transfected with 2 μg of YAP plasmid, 0.5 μg of HA-K48-Ub or HA-K48R-Ub, and either 0.5 μg of Flag-tag or Flag-JOSD1 plasmids. Subsequently, the cells were immunoblotted with specific antibodies. YAP is deubiquitinated by JOSD1 through K48-linked polyubiquitination. D Reducing JOSD1 levels led to a higher amount of polyubiquitinated YAP. HCT116 cells were transfected with 0.5 μg HA-Ub plasmid and 20uM JOSD1 siRNA after treatment with MG132 for 6 h, followed by immunoblotting with specified antibodies. E, F JOSD1 deficiency increased YAP polyubiquitination related to K48, but not K48R. HCT116 cells were transfected with 0.5 μg HA-K48-Ub or HA-K48R-Ub plasmid and 20uM siJOSD1 after treatment with MG132 for 6 h, followed by immunoblotting with specified antibodies. G, H The JOSD1 mutant lacking deubiquitinating enzyme activity cannot enhance the accumulation of polyubiquitinated YAP. HEK-293T cells were transfected with 2 μg YAP plasmid, 0.5 μg HA-Ub/HA-K48 Ub plasmid and 0.5 μg Flag-tag or Flag-JOSD1 or Flag-JOSD1C36A upon MG132 treatment for 6 h and then immunoblotted with the indicated antibodies. I, J JOSD1 deubiquitinates YAP via its N-terminal region. HEK-293T cells were transfected with 2 μg YAP plasmid, 0.5 μg HA-Ub/HA-K48 Ub plasmid, and 0.5 μg EGFP-tag or EGFP-JOSD1 full-length or deletion mutant plasmids after 6-h MG132 treatment. Subsequently, immunoblotting was performed using specified antibodies.