Fig. 4.
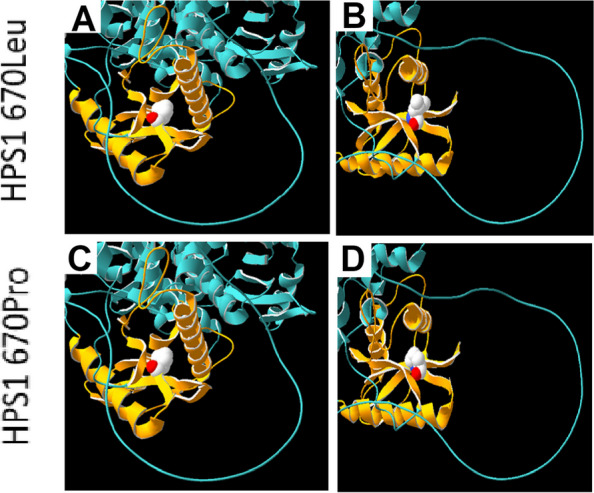
Protein modelling studies (Swiss model, SPDBV v4.10) demonstrating the location of the HPS1 670Leu position within the C-terminal Fuz-longin-3 domain (Third Longin domain of FUZ, MON1 and HPS1, orange) [47, 48]. Structurally this domain is composed of an α/β fold which contains five anti-parallel β-strands organised as a central β-sheet, with two α-helices around it (Sanchez-Pulido & Ponting, 2020). (A, B) demonstrate the reference amino acid Leucine and (C, D) show the alternate Proline arising from the novel HPS1 variant (NM_000195.5: c.2009 T > C; p.(Leu670Pro)). The location of this variant within the central β-sheet is likely to affect the function of this domain in Rab signalling
