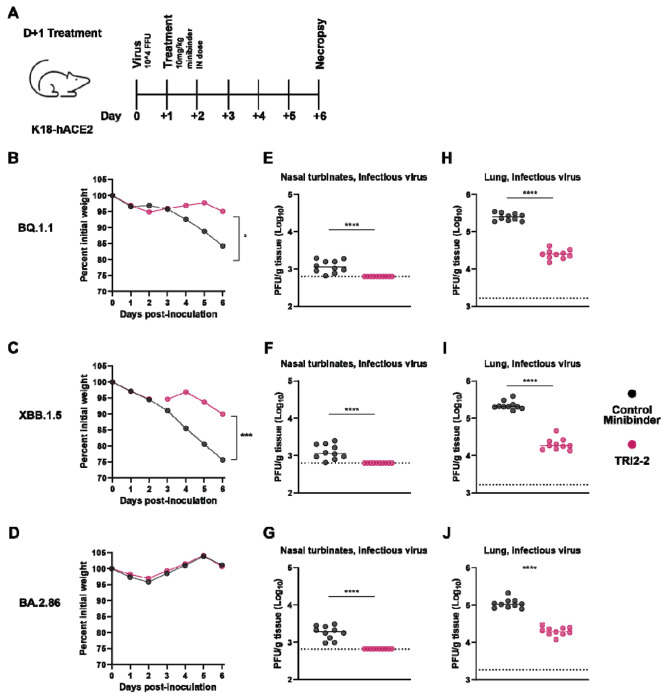
Figure 2. Post-exposure TRI2-2 administration protects mice challenged with the SARS-CoV-2 BQ.1.1, XBB.1.5, and BA.2.86 variants.
(A) Schematic of study design. (B-D) Weight loss for mice challenged with BQ.1.1 (B), XBB.1.5 (C), or BA.2.86 (D) (dots represent mean.; n=10 mice per group per challenge virus; differences in area under the curves assessed by Student’s t-test with Welch’s correction for each virus; *P < 0.01; ***P<0.001). (E-G) Nasal turbinate infectious viral titers for mice challenged with BQ.1.1 (E), XBB.1.5 (F), or BA.2.86 (G). (H-J) Lung infectious viral titers for mice challenged with BQ.1.1 (H), XBB.1.5 (I), or BA.2.86 (J) (lines indicate median.; n=10 mice per group per challenge virus, two experiments; Two-tailed Mann-Whitney test between control and TRI2-2 treatment; ns, not significant; *P < 0.05, ***P < 0.001, ****P < 0.0001.).