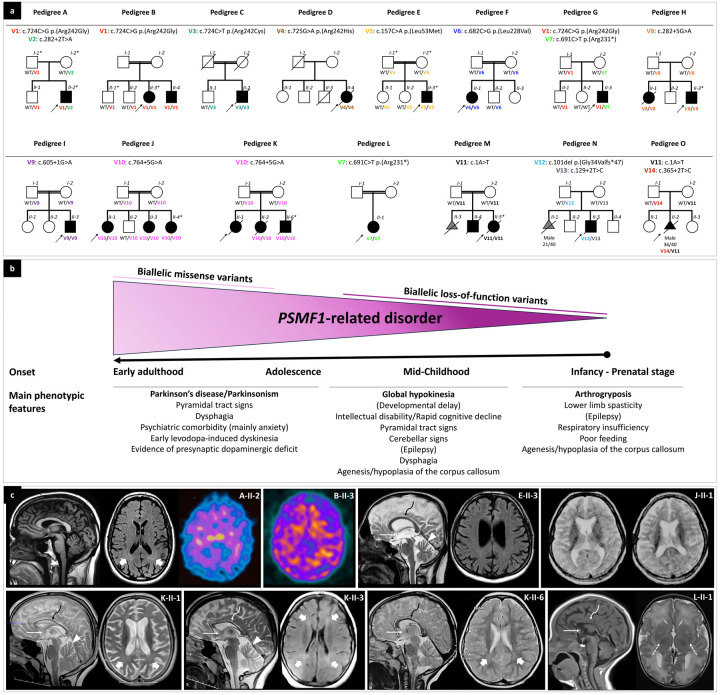
Figure 1. Partial pedigrees and phenotypic features of individuals with biallelic PSMF1 variants.
(a) Partial pedigrees of PSMF1 families. Simplified pedigrees of 15 unrelated PSMF1 families are presented, with the results of segregation analysis of PSMF1 variants. In the diagrams, males are represented by squares, females by circles and fetuses by triangles. Fetal gender and gestational age at death (n weeks/40 weeks) are reported below their symbol, if available. Consanguineous marriages are denoted by double lines between symbols, while probands are marked with an arrow. Individuals exhibiting a neurological disease phenotype are represented by black filled symbols. Fetuses aborted for undetermined cause (M-II-3, N-II-1) are represented by a gray filled symbol. Roman numerals indicate generations, whereas Arabic numbers denote individuals within each generation. Variants are referenced to PSMF1 transcript NM_006814.5 and displayed with different font colors (see Fig. 2a and Supplementary File 1). “WT” designates the wild-type PSMF1 allele. An asterisk identifies dermal fibroblast donors. Fully identifying pedigrees are available upon request to the corresponding author. (b) Genotype-phenotype correlation in PSMF1-related disorder. Schematic representation of the core phenotypic features associated with biallelic variants in PSMF1, with indication of age at onset and main clinical manifestations in subgroups of subjects (see main text). Increasingly severe phenotypes correspond to progressively more deleterious mutational effects. (c) Neuroimaging features of PSMF1-related disorder. Brain MRI with sagittal T1- or T2-weighted images and axial FLAIR or T2-weighted images of individuals A-II-2 (25–30 years), E-II-3 (25–30 years), J-II-1 (10–15 years), K-II-1 (10–15 years), K-II-3 (10–15 years), K-II-6 (0–5 years) and L-II-1 (infancy). Mild-to-moderate enlargement of the cerebral subarachnoid spaces is noted in subjects A-II-2, E-II-3, J-II-1, K-II-1, and K-II-3. Enlargement of the subarachnoid spaces of the superior cerebellar vermis is present in individuals E-II-3, K-II-1, and K-II-3. Additional faint T2/FLAIR-signal alterations are visible in the periventricular white matter in individuals A-II-2, K-II-1, K-II-3, and K-II-6 (thick arrows). There is hypoplasia of corpus callosum (curved arrows) and small anterior commissure (thin arrows) in subjects E-II-3, J-II-1, K-II-1, K-II-3, K-II-6, and L-II-1. There is lack of myelination of the posterior limbs of the internal capsules in patient L-II-1 (dashed arrows), associated with foci of T2 hyperintensity at the level of the putamina. DaTscan of subjects A-II-2 and B-II-3 reveals severely reduced tracer uptake in the striatum bilaterally, with increased background activity.