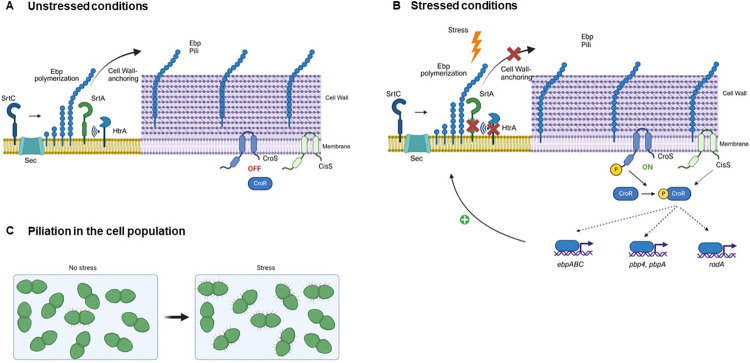
Fig 8. Model integrating the HtrA quality control system and the CroRS signalling pathway into pilus biogenesis.
(A) During unstressed conditions, pilus monomers are exported through the Sec pathway, then the sortase C (SrtC) enzyme polymerizes EbpA (tip) and EbpC (shaft) subunits to full pilus length and finally links the structure to the EbpB (base) which remains transiently anchored to the cell membrane [28]. Then, the sortase A (SrtA) enzyme cleaves the CWS signal of EbpB and efficiently anchors the pilus to the cell wall [28,29]. Two models have been proposed for SrtA-dependent pili processing in E. faecalis [28,29]. The HtrA bifunctional chaperone/protease monitors pilus processing by SrtA and contributes to clearance of sporadic aberrant pili. (B) Certain environmental stresses (e.g. stomach pH, gut bile salts, immune oxidative burst) cause protein misfolding and aggregation, impairing successful processing by SrtA. If HtrA activation is insufficient to cope with the proteotoxic stress, aberrant proteins accumulate and perturb the cell membrane, leading to activation of the CroS histidine kinase. CroS in turn activates the CroR response regulator, which regulates transcription of pilus (ebpABC), penicillin-binding proteins (pbp4, pbpA), and peptidoglycan-active (rodA) genes among others. In some E. faecalis strains, the TCS CisRS can phosphorylate CroR [49]. Dotted arrows indicate confirmed indirect regulation (pbpA) [76] or unknown direct or indirect regulation by CroR. Induction of ebpABC genes leads to hyper-piliation, while repression of rodA partially leads to cell morphology alterations. (C) During non-stressed conditions, only a small percentage of cells expresses surface-exposed pili [2,4]. However, perturbation of the membrane due to accumulation of aberrant proteins during stress leads to piliation of a higher number of cells. Figure created with BioRender.com.